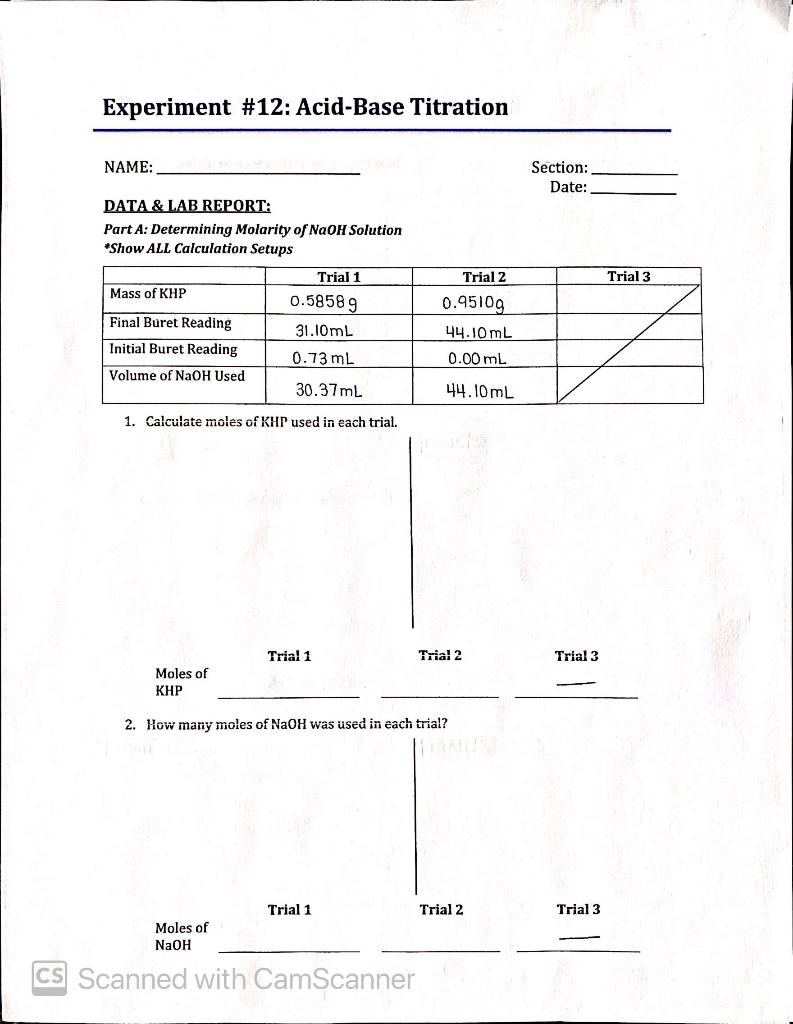
Ap Worksheet 04F Titration Calculations Answers . Titration calculations & answers 1. Ap worksheet 4f (titration) 1. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Use the information to determine the concentration of the hydrochloric acid. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Buffer solution, common ion, conjugate base/acid, equivalence. The following might indicate the question deals with buffers and/or titrations: All stages are covered and the. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3.
from www.vrogue.co
Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Buffer solution, common ion, conjugate base/acid, equivalence. Use the information to determine the concentration of the hydrochloric acid. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. All stages are covered and the. Titration calculations & answers 1. Ap worksheet 4f (titration) 1. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. The following might indicate the question deals with buffers and/or titrations:
Acid Base Titration Lab Answers Answerdata vrogue.co
Ap Worksheet 04F Titration Calculations Answers All stages are covered and the. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Titration calculations & answers 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. The following might indicate the question deals with buffers and/or titrations: Use the information to determine the concentration of the hydrochloric acid. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Ap worksheet 4f (titration) 1. Buffer solution, common ion, conjugate base/acid, equivalence. All stages are covered and the.
From worksheetzonegibson55.z21.web.core.windows.net
Titration Worksheet Answer Key Ap Worksheet 04F Titration Calculations Answers Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. All stages are covered and the. Ap worksheet 4f (titration) 1. Buffer solution, common ion, conjugate base/acid, equivalence. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. A student is given a 25.0. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Free titrations practice worksheet answers, Download Free titrations Ap Worksheet 04F Titration Calculations Answers A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. The following might indicate the question deals with buffers and/or titrations: Use the information to determine the concentration of the hydrochloric acid. Answer is communicated coherently and shows a logical progression from stage. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titration Calculations Worksheet Professor Feito Twin vrogue.co Ap Worksheet 04F Titration Calculations Answers Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. All stages are covered and the. Titration calculations & answers 1. Use the. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Ap Worksheet 04F Titration Calculations Answers Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. All stages are covered and the. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Buffer solution, common ion, conjugate base/acid, equivalence. The following might indicate the question deals with buffers. Ap Worksheet 04F Titration Calculations Answers.
From answermueller.z21.web.core.windows.net
Acid Base Titration Calculation Worksheet Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Ap worksheet 4f (titration) 1. All stages are covered and the. Buffer solution, common ion, conjugate base/acid, equivalence. The following might indicate. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Ap Worksheet 04F Titration Calculations Answers Titration calculations & answers 1. All stages are covered and the. Ap worksheet 4f (titration) 1. Buffer solution, common ion, conjugate base/acid, equivalence. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Simple titration calculations since the equivalence point is when the number of moles of acid and base. Ap Worksheet 04F Titration Calculations Answers.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. The following might indicate the question deals with buffers and/or titrations: All stages are covered and the. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Buffer solution, common ion, conjugate base/acid, equivalence. Ap worksheet. Ap Worksheet 04F Titration Calculations Answers.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Ap Worksheet 04F Titration Calculations Answers Ap worksheet 4f (titration) 1. Buffer solution, common ion, conjugate base/acid, equivalence. Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. All stages are covered and the. Answer is communicated coherently and shows. Ap Worksheet 04F Titration Calculations Answers.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Buffer solution, common ion, conjugate base/acid, equivalence. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Titration calculations & answers 1. Ap worksheet 4f (titration) 1. All stages are covered and the. A student is given a 25.0 ml sample. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Ap Worksheet 04F Titration Calculations Answers Buffer solution, common ion, conjugate base/acid, equivalence. Ap worksheet 4f (titration) 1. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. The following might. Ap Worksheet 04F Titration Calculations Answers.
From www.studocu.com
Examples of back titration w answers 2008 MCHE223 Studocu Ap Worksheet 04F Titration Calculations Answers Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Use the information to determine the concentration of the hydrochloric acid. The following might indicate the question deals with buffers and/or titrations:. Ap Worksheet 04F Titration Calculations Answers.
From dorkiestenews.blogspot.com
Acid Base Titration Worksheet Answers Acid Base Titration Worksheet Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. All stages are covered and the. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Name Acid Base Titration Lab Worksheet Titration Cheg vrogue.co Ap Worksheet 04F Titration Calculations Answers Ap worksheet 4f (titration) 1. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Use the information to determine the concentration of the hydrochloric acid. All stages are covered and the. Buffer solution, common ion, conjugate base/acid, equivalence. Answer is communicated coherently. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Ap Worksheet 04F Titration Calculations Answers Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. The following might indicate the question deals with buffers and/or titrations: Use the information to determine the concentration of the hydrochloric acid. Buffer solution, common ion, conjugate base/acid, equivalence. A student is given a 25.0 ml sample of. Ap Worksheet 04F Titration Calculations Answers.
From lessondbspermatics.z13.web.core.windows.net
Acid And Base Calculations Worksheets Ap Worksheet 04F Titration Calculations Answers Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Use the information to determine the concentration of the hydrochloric acid. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. The following might indicate the question deals with buffers and/or titrations:. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Free Printable Acids and Bases Titration Worksheets Worksheets Library Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. The following might indicate the question deals with buffers and/or titrations: Buffer solution, common ion, conjugate base/acid, equivalence. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it. Ap Worksheet 04F Titration Calculations Answers.
From mungfali.com
Titration Worksheet Ap Worksheet 04F Titration Calculations Answers All stages are covered and the. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Ap worksheet 4f (titration) 1. Titration calculations. Ap Worksheet 04F Titration Calculations Answers.
From materiallibrarysevert.z21.web.core.windows.net
Neutralization And Titration Worksheet Ap Worksheet 04F Titration Calculations Answers Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3.. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Acid Base Titrations Worksheet Worksheets Library Ap Worksheet 04F Titration Calculations Answers Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Use the information to determine the concentration of the hydrochloric acid. Buffer solution, common ion, conjugate base/acid, equivalence. Titration calculations & answers 1. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml. Ap Worksheet 04F Titration Calculations Answers.
From davida.davivienda.com
Titration Problems Worksheet Answers Printable Word Searches Ap Worksheet 04F Titration Calculations Answers Ap worksheet 4f (titration) 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Titration calculations & answers 1. All stages are covered and the. Use the information to determine the concentration of the hydrochloric acid. Calculate the concentration of a phosphoric acid solution if 45.0 ml. Ap Worksheet 04F Titration Calculations Answers.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Ap Worksheet 04F Titration Calculations Answers All stages are covered and the. Use the information to determine the concentration of the hydrochloric acid. Ap worksheet 4f (titration) 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then. Ap Worksheet 04F Titration Calculations Answers.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Ap Worksheet 04F Titration Calculations Answers The following might indicate the question deals with buffers and/or titrations: Ap worksheet 4f (titration) 1. All stages are covered and the. Use the information to determine the concentration of the hydrochloric acid. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. A student is given a 25.0 ml sample of. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Acid Base Titration Lab Answers Answerdata vrogue.co Ap Worksheet 04F Titration Calculations Answers Buffer solution, common ion, conjugate base/acid, equivalence. Ap worksheet 4f (titration) 1. Use the information to determine the concentration of the hydrochloric acid. The following might indicate the question deals with buffers and/or titrations: Titration calculations & answers 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can. Ap Worksheet 04F Titration Calculations Answers.
From philo-khosravani.blogspot.com
Acid Base Titration Worksheet Answers philokhosravani Ap Worksheet 04F Titration Calculations Answers Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Titration calculations & answers 1. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titrations Home Learning Worksheet Gcse Teaching Reso vrogue.co Ap Worksheet 04F Titration Calculations Answers Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Titration calculations & answers 1. Ap worksheet 4f (titration) 1. All stages are covered and the. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Simple titration calculations since the equivalence. Ap Worksheet 04F Titration Calculations Answers.
From www.scribd.com
Titration Calculation Answer Key PDF Ph Chemistry Ap Worksheet 04F Titration Calculations Answers The following might indicate the question deals with buffers and/or titrations: A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Use the information to determine the concentration of the hydrochloric acid. Buffer solution, common ion, conjugate base/acid, equivalence. Simple titration calculations since. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
acid base titration worksheet answer key Worksheets Library Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. The following might indicate the question deals with buffers and/or titrations: All stages are covered and the. A student is given a 25.0 ml sample of a. Ap Worksheet 04F Titration Calculations Answers.
From www.hotzxgirl.com
Titration Calculations Made Easy How To Do Titration Calculations Hot Ap Worksheet 04F Titration Calculations Answers Ap worksheet 4f (titration) 1. All stages are covered and the. Titration calculations & answers 1. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Use the information to determine the concentration of the hydrochloric acid. Calculate the concentration of a phosphoric acid solution if 45.0 ml. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Acid Base Titration Worksheet Answers W336 Titrations vrogue.co Ap Worksheet 04F Titration Calculations Answers Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration. Ap Worksheet 04F Titration Calculations Answers.
From www.vrogue.co
Titration Calculations Worksheet Professor Feito Twin vrogue.co Ap Worksheet 04F Titration Calculations Answers A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. The following might indicate the question deals with buffers and/or titrations: Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make.. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Weak Acid Base Titration worksheet Weak Acid Base Titration Ap Worksheet 04F Titration Calculations Answers Buffer solution, common ion, conjugate base/acid, equivalence. Use the information to determine the concentration of the hydrochloric acid. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of.. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Acid base titration worksheet Live Worksheets Worksheets Library Ap Worksheet 04F Titration Calculations Answers Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Simple titration calculations since the equivalence point is when the number of moles of acid and base are equal, we can make. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine. Ap Worksheet 04F Titration Calculations Answers.
From worksheets.clipart-library.com
Solved Titration calculations worksheet 1. You measured out Ap Worksheet 04F Titration Calculations Answers The following might indicate the question deals with buffers and/or titrations: Calculate the concentration of a phosphoric acid solution if 45.0 ml of it were required to neutralize 20.8 ml of. Use the information to determine the concentration of the hydrochloric acid. All stages are covered and the. Buffer solution, common ion, conjugate base/acid, equivalence. A student is given a. Ap Worksheet 04F Titration Calculations Answers.
From www.scribd.com
titration_answer_keys PDF Ap Worksheet 04F Titration Calculations Answers Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. The following might indicate the question deals with buffers and/or titrations: Buffer solution, common ion, conjugate base/acid, equivalence. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage 3. Simple titration calculations since the equivalence point is. Ap Worksheet 04F Titration Calculations Answers.
From studylib.net
Titration Worksheet Ap Worksheet 04F Titration Calculations Answers The following might indicate the question deals with buffers and/or titrations: Titration calculations & answers 1. A student is given a 25.0 ml sample of a solution of an unknown monoprotic acid and asked to determine the concentration of the acid by titration. Answer is communicated coherently and shows a logical progression from stage 1 to stage 2 then stage. Ap Worksheet 04F Titration Calculations Answers.