Sop Equipment Cleaning . The general rules and gmp requirements. All cleaners should undergo induction. How to develop cleaning standard operating procedures? The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. Standard operating procedure for cleaning of manufacturing equipment. The rules and gmp requirements apply equally to contract cleaners and operations staff. Transform your cleaning sops into actionable digital. The purpose of this sop is to establish a. What are cleaning standard operating procedures (sops)? The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as.
from studylib.net
The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. Transform your cleaning sops into actionable digital. The purpose of this sop is to establish a. Standard operating procedure for cleaning of manufacturing equipment. All cleaners should undergo induction. The rules and gmp requirements apply equally to contract cleaners and operations staff. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The general rules and gmp requirements. How to develop cleaning standard operating procedures?
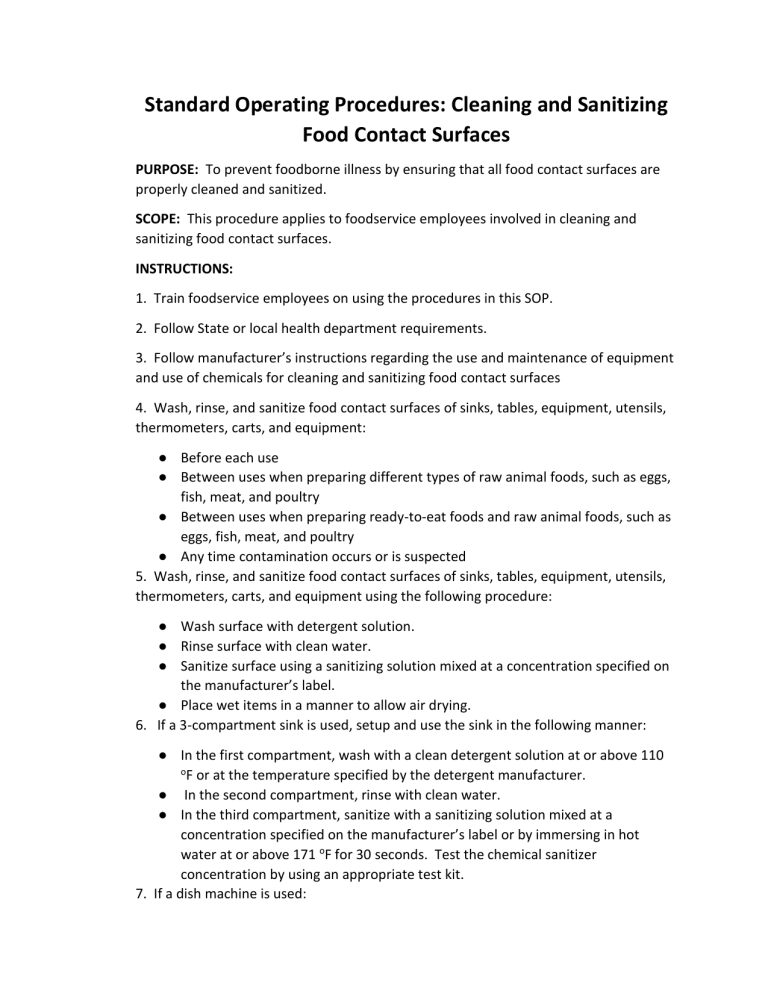
SOP cleaning and sanitizing 2 15 16
Sop Equipment Cleaning All cleaners should undergo induction. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The rules and gmp requirements apply equally to contract cleaners and operations staff. How to develop cleaning standard operating procedures? All cleaners should undergo induction. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. Standard operating procedure for cleaning of manufacturing equipment. What are cleaning standard operating procedures (sops)? The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The purpose of this sop is to establish a. The general rules and gmp requirements. Transform your cleaning sops into actionable digital.
From id.scribd.com
SOP Cleaning Service PDF Sop Equipment Cleaning The purpose of this sop is to establish a. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records.. Sop Equipment Cleaning.
From aps-online.net
Standard Operating Procedure (SOP) and Work Instruction (WI) Writing Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. Standard operating procedure for cleaning of manufacturing equipment. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. Transform your cleaning sops into actionable digital. What are cleaning standard operating procedures (sops)? The four key. Sop Equipment Cleaning.
From www.monogramcleanforce.com
Monogram Clean Force All Surface Floor Cleaner Floor Cleaning Procedures Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The general rules and gmp requirements. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The rules and gmp requirements apply equally to contract cleaners and operations staff. Standard. Sop Equipment Cleaning.
From www.scribd.com
SOP OF Operating and Cleaning Procedure For Vacuum Cleaner PDF Sop Equipment Cleaning Standard operating procedure for cleaning of manufacturing equipment. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and. Sop Equipment Cleaning.
From www.scribd.com
SOP Cleaning Services PDF Sop Equipment Cleaning The general rules and gmp requirements. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. How to develop cleaning standard operating procedures? What are cleaning standard operating procedures (sops)? The rules and gmp requirements apply equally to contract cleaners and operations staff. Standard operating procedure for cleaning of manufacturing. Sop Equipment Cleaning.
From www.scribd.com
SOP of Development and Validation of Analytical Method For Equipment Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. Standard operating procedure for cleaning of manufacturing equipment. What are cleaning standard operating procedures (sops)? The general rules and gmp requirements. The rules and gmp requirements apply equally to contract cleaners and operations staff. The purpose of this standard operating. Sop Equipment Cleaning.
From pharmajobswalkin.com
SOP On Cleaning Procedure For New Equipment, Machineries, And Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The purpose of this sop is to establish a. Transform your cleaning sops into actionable digital. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The purpose of this standard operating procedure (sop) is. Sop Equipment Cleaning.
From learngxp.com
6 Key Requirements of a Cleaning Process SOP [Video] LearnGxP Sop Equipment Cleaning Standard operating procedure for cleaning of manufacturing equipment. Transform your cleaning sops into actionable digital. How to develop cleaning standard operating procedures? The purpose of this sop is to establish a. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The rules and gmp requirements apply equally to contract. Sop Equipment Cleaning.
From berkasbelajar.github.io
Sop Cleaning Berkas Belajar Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. How to develop cleaning standard operating procedures? The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The general. Sop Equipment Cleaning.
From www.slideserve.com
PPT Understanding Decontamination SOP’s PowerPoint Presentation, free Sop Equipment Cleaning How to develop cleaning standard operating procedures? What are cleaning standard operating procedures (sops)? Standard operating procedure for cleaning of manufacturing equipment. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The sop. Sop Equipment Cleaning.
From setupmyhotel.com
SOP Kitchen / F&B Production Equipment Handling, Cleaning and Sop Equipment Cleaning The rules and gmp requirements apply equally to contract cleaners and operations staff. How to develop cleaning standard operating procedures? Transform your cleaning sops into actionable digital. The general rules and gmp requirements. The purpose of this sop is to establish a. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of. Sop Equipment Cleaning.
From pharmastate.academy
SOP FOR OPERATION AND CLEANING OF COATING PAN Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. Standard operating procedure for cleaning of manufacturing equipment. How to develop cleaning standard operating procedures? All cleaners should undergo induction. The rules and gmp requirements apply equally to contract cleaners and operations staff. What are cleaning standard operating procedures (sops)? The general rules and gmp. Sop Equipment Cleaning.
From studylib.net
Cleaning and Sanitizing Food Contact Surfaces SOP Sop Equipment Cleaning The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. Standard operating procedure for cleaning of manufacturing equipment. The general rules and gmp requirements. The rules and gmp requirements apply equally to contract cleaners. Sop Equipment Cleaning.
From id.scribd.com
SOP Cleaning Service.docx Sop Equipment Cleaning The purpose of this sop is to establish a. Transform your cleaning sops into actionable digital. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. What are cleaning standard operating procedures (sops)? Standard operating procedure for cleaning of manufacturing equipment. The four key elements of equipment cleaning in gmp. Sop Equipment Cleaning.
From www.scribd.com
SOP for Operation and Cleaning of Conveyer Belt Personal Protective Sop Equipment Cleaning The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The purpose of this. Sop Equipment Cleaning.
From www.scribd.com
SOP For Cleaning of Equipment and Accessories in Production Area Sop Equipment Cleaning The rules and gmp requirements apply equally to contract cleaners and operations staff. Standard operating procedure for cleaning of manufacturing equipment. Transform your cleaning sops into actionable digital. How to develop cleaning standard operating procedures? The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions. Sop Equipment Cleaning.
From www.sampletemplates.com
FREE 61+ SOP Templates in PDF MS Word Sop Equipment Cleaning How to develop cleaning standard operating procedures? Standard operating procedure for cleaning of manufacturing equipment. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. All cleaners should undergo induction. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The four key elements of. Sop Equipment Cleaning.
From setupmyhotel.com
Types of Cleaning Procedures in Hotel Housekeeping SetupMyHotel Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. How to develop cleaning standard operating procedures? The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining. Sop Equipment Cleaning.
From www.scribd.com
Sop For Operation & Cleaning of Bottle Filling Machine PDF Sop Equipment Cleaning How to develop cleaning standard operating procedures? Standard operating procedure for cleaning of manufacturing equipment. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. All cleaners should undergo induction. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. What are cleaning standard operating. Sop Equipment Cleaning.
From www.scribd.com
SOP Cleaning and Sanitizing Sop Equipment Cleaning Transform your cleaning sops into actionable digital. All cleaners should undergo induction. How to develop cleaning standard operating procedures? The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. Standard operating procedure for cleaning of manufacturing equipment. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of. Sop Equipment Cleaning.
From ruangilmu.github.io
Sop Cleaning Ruang Ilmu Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. How to develop cleaning standard operating procedures? The general rules and gmp requirements. Standard operating procedure for cleaning of manufacturing equipment. What are cleaning standard operating procedures (sops)? Transform your cleaning sops into actionable digital. The four key elements of equipment cleaning in gmp are. Sop Equipment Cleaning.
From templatelab.com
37 Best Standard Operating Procedure (SOP) Templates Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The general rules and gmp requirements. What are cleaning standard operating procedures (sops)? The purpose of this sop is to establish a. Standard operating procedure for cleaning of manufacturing equipment. The sop for equipment maintenance and cleaning provides a comprehensive. Sop Equipment Cleaning.
From urstay.co.uk
5 STEP CLEANING PROCESS UR STAY SERVICED APARTMENTS Sop Equipment Cleaning The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. How to develop cleaning standard operating procedures? Transform your cleaning sops into actionable digital. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The general rules and gmp requirements. All cleaners should undergo induction.. Sop Equipment Cleaning.
From www.scribd.com
SOP On Cleaning and Sanitization in Pharmaceuticalljhiu PDF Safety Sop Equipment Cleaning What are cleaning standard operating procedures (sops)? The purpose of this sop is to establish a. How to develop cleaning standard operating procedures? All cleaners should undergo induction. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and. Sop Equipment Cleaning.
From berkasbelajar.github.io
Sop Cleaning Berkas Belajar Sop Equipment Cleaning Transform your cleaning sops into actionable digital. The purpose of this sop is to establish a. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The general rules and gmp requirements. The sop. Sop Equipment Cleaning.
From www.scribd.com
SOP For Cleaning and Sanitation of Microbiology Section With Sop Equipment Cleaning The purpose of this sop is to establish a. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. How to develop cleaning standard operating procedures? The rules and gmp requirements apply equally to. Sop Equipment Cleaning.
From www.researchgate.net
Standard operating procedure. PPE, personal protective equipment; SOP Sop Equipment Cleaning What are cleaning standard operating procedures (sops)? The purpose of this sop is to establish a. Standard operating procedure for cleaning of manufacturing equipment. How to develop cleaning standard operating procedures? The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The general rules and gmp requirements. The purpose of this standard operating procedure (sop). Sop Equipment Cleaning.
From pharmadekho.com
sop for Preventive Maintenance of Linear Bottle Washing Machine Sop Equipment Cleaning The rules and gmp requirements apply equally to contract cleaners and operations staff. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which. Sop Equipment Cleaning.
From www.gofmx.com
Maintenance SOP FMX Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. All cleaners should undergo induction. The rules and gmp requirements apply equally to contract cleaners and operations staff. What are cleaning standard operating procedures (sops)? How to develop cleaning standard operating procedures? The four key elements of equipment cleaning in. Sop Equipment Cleaning.
From studylib.net
SOP cleaning and sanitizing 2 15 16 Sop Equipment Cleaning All cleaners should undergo induction. How to develop cleaning standard operating procedures? The rules and gmp requirements apply equally to contract cleaners and operations staff. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The purpose of this sop is to establish a. The sop for equipment maintenance and. Sop Equipment Cleaning.
From www.fooddocs.com
Sanitation Standard Operating Procedures (SSOP) Free Download Sop Equipment Cleaning The general rules and gmp requirements. How to develop cleaning standard operating procedures? The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The purpose of this sop is to establish a. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods. Sop Equipment Cleaning.
From id.scribd.com
SOP Cleaning Service PDF Sop Equipment Cleaning The purpose of this sop is to establish a. The sop for equipment maintenance and cleaning provides a comprehensive framework for routine and preventive. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in. Sop Equipment Cleaning.
From franchisetraining.co.za
Cleaning SOP and Equipment Management. Franchise Training Sop Equipment Cleaning The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. The purpose of this. Sop Equipment Cleaning.
From pharmaceuticalcarrier.com
Standard Operating Procedure (SOP) for Cleaning of Dehumidifier Sop Equipment Cleaning 5.1.3 clean the major equipment (like rmg, octagonal blender, fbd etc.), which are immovable (clean in place) of installation as. The purpose of this standard operating procedure (sop) is to establish procedures for the cleaning and sanitization of manufacturing equipment. What are cleaning standard operating procedures (sops)? The general rules and gmp requirements. Transform your cleaning sops into actionable digital.. Sop Equipment Cleaning.
From www.scribd.com
SOP Cleaning Service Sop Equipment Cleaning All cleaners should undergo induction. How to develop cleaning standard operating procedures? Standard operating procedure for cleaning of manufacturing equipment. The four key elements of equipment cleaning in gmp are standardizing written cleaning procedures, validating the cleaning methods to ensure effectiveness, specifying optimal cleaning conditions like proper cleaning agents and temperatures, and maintaining detailed cleaning records. Transform your cleaning sops. Sop Equipment Cleaning.