Beer Lambert Law For Kmno4 . In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
from slidetodoc.com
In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
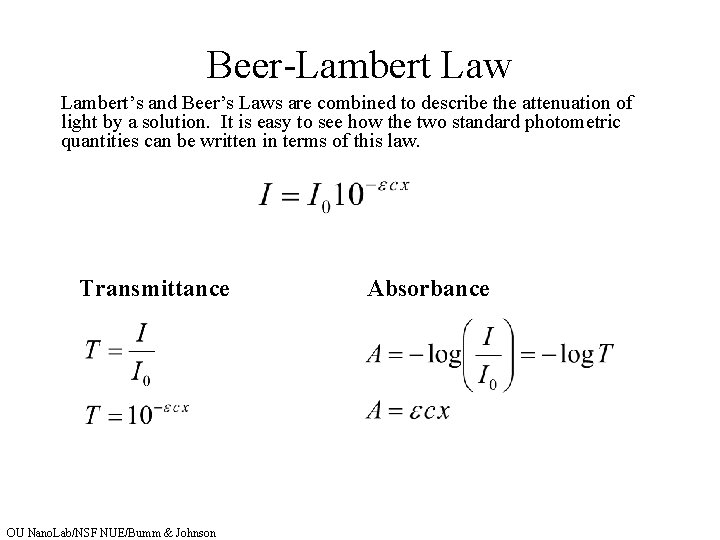
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers
Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law For Kmno4 You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Experiment to verify lambert beer s law for kmno4 colorimetrically Studypool Beer Lambert Law For Kmno4 You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.youtube.com
Determine the concentration of given kmno4 soln beer's Lambert law..... by experimental Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law For Kmno4.
From www.youtube.com
Determining Concentration of Unknown KMnO4 Solution by UVVisible Spectroscopy & BeerLambert's Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law For Kmno4.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Determination of age composition of kmno4 k2cr2o7 in given solution by spectrometry Beer Lambert Law For Kmno4 You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.scribd.com
Beer S Law Plot of KMnO4 PDF PDF Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law AP Chemistry Khan Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Experiment to verify lambert beer s law for kmno4 colorimetrically Studypool Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From slideplayer.com
The MichaelisMenton Model ppt download Beer Lambert Law For Kmno4 You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Beer Lambert Law For Kmno4 You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.youtube.com
To verify Lambert Beers law for KMnO4 using colorimeter & to determine conc. of unknown solution Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From brainly.in
Verify the Beer Lambert law for KMnO4 and determine concentration of the given solution of the Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law For Kmno4 You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified... Download Scientific Diagram Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer Lambert Law For Kmno4.
From www.chegg.com
Solved BeerLambert Law Application Example 1 A compound Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.numerade.com
SOLVED Lab Report Beer's Law Part I Determination of the Wavelength of Maximum Absorbance Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.numerade.com
SOLVED Verify the Beer Lambert law for KMnO4 and determine concentration of the given solution Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From allbachelor.com
Verify Lambert’s Beer’s Law & Determine KMnO4 Concentration All Bachelor Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Beer lambert law Studypool Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From allbachelor.com
Verify Lambert’s Beer’s Law & Determine KMnO4 Concentration All Bachelor Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Experiment to verify lambert beer s law for kmno4 colorimetrically Studypool Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Beer lambert law Studypool Beer Lambert Law For Kmno4 In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law For Kmno4.
From www.chegg.com
Solved Concentration Determination of KMnO4 Solution by Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Theory the primary objective of this experiment is to determine the. Beer Lambert Law For Kmno4.
From www.studypool.com
SOLUTION Experiment to verify lambert beer s law for kmno4 colorimetrically Studypool Beer Lambert Law For Kmno4 Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Theory the primary objective of this experiment is to determine the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer Lambert Law For Kmno4.
From www.scribd.com
Verification of BeerLambert's Law and Determination of an Unknown Concentration of Potassium Beer Lambert Law For Kmno4 Theory the primary objective of this experiment is to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. Beer Lambert Law For Kmno4.