Magnesium Insoluble Water . The hydroxides of transition metals and. Water solubility of magnesium hydroxide is 12 mg/l. Magnesium hydroxide appears to be insoluble in water. Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. However, if it is shaken in water and filtered, the solution is slightly basic. Find the molar solubility of a compound in water with the solubility calculator, rules or a table.
from stock.adobe.com
Find the molar solubility of a compound in water with the solubility calculator, rules or a table. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. However, if it is shaken in water and filtered, the solution is slightly basic. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Water solubility of magnesium hydroxide is 12 mg/l. Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of transition metals and. Magnesium hydroxide appears to be insoluble in water. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the.
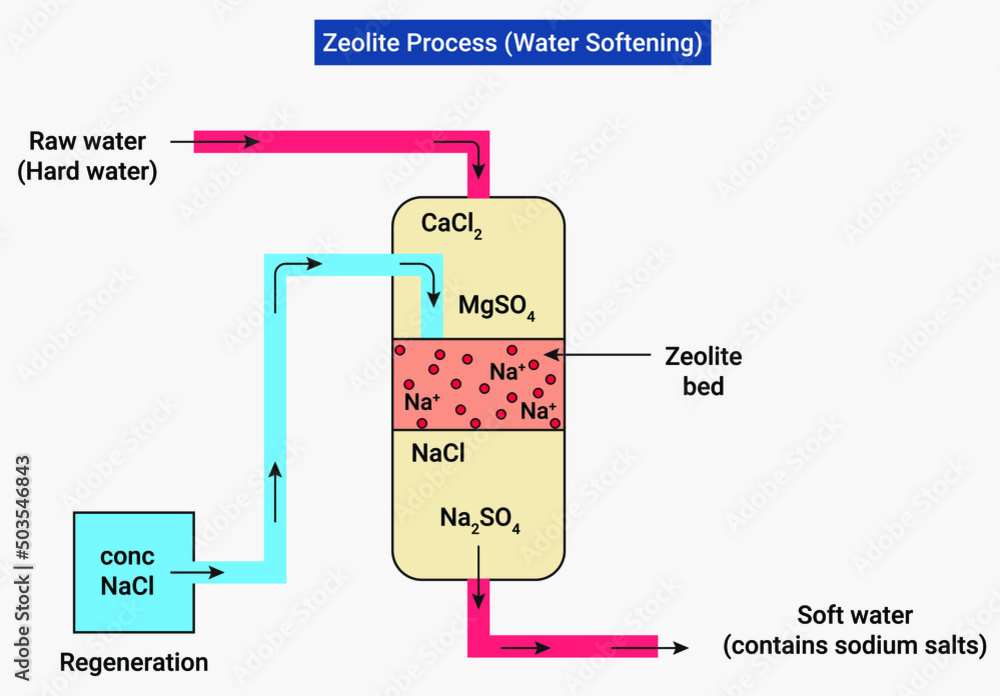
The zeolite softening process is used for removing both the temporary and permanent hardness of
Magnesium Insoluble Water 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. The hydroxides of transition metals and. Water solubility of magnesium hydroxide is 12 mg/l. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Other magnesium compounds are clearly more water soluble, for example magnesium. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Magnesium hydroxide appears to be insoluble in water.
From signalticket9.pythonanywhere.com
Glory What Happens When Magnesium Reacts With Steam Formula Of Time Period Magnesium Insoluble Water Other magnesium compounds are clearly more water soluble, for example magnesium. However, if it is shaken in water and filtered, the solution is slightly basic. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The hydroxides of transition metals and. Water solubility of magnesium hydroxide is 12 mg/l. In. Magnesium Insoluble Water.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O YouTube Magnesium Insoluble Water Find the molar solubility of a compound in water with the solubility calculator, rules or a table. The hydroxides of transition metals and. Other magnesium compounds are clearly more water soluble, for example magnesium. Magnesium hydroxide appears to be insoluble in water. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium. Magnesium Insoluble Water.
From webmis.highland.cc.il.us
Aqueous Solutions Magnesium Insoluble Water Other magnesium compounds are clearly more water soluble, for example magnesium. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The hydroxides of alkaline earth metals, like magnesium. Magnesium Insoluble Water.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Insoluble Water Water solubility of magnesium hydroxide is 12 mg/l. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. However, if it is shaken in water and filtered, the solution is slightly basic. Magnesium hydroxide appears to be insoluble in water. The hydroxides of transition metals and. The hydroxides of alkaline. Magnesium Insoluble Water.
From lazyplant.com
Water is a natural source of Magnesium! LazyPlant Magnesium Insoluble Water Find the molar solubility of a compound in water with the solubility calculator, rules or a table. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are.. Magnesium Insoluble Water.
From timehealth.co.uk
Marine Magnesium Purified Sea Water Minerals & Micronutrients 308mg of Magnesium 120 Capsules Magnesium Insoluble Water In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Water solubility of magnesium hydroxide is 12 mg/l. Other magnesium compounds are clearly more water soluble, for example magnesium. Magnesium hydroxide appears to be insoluble in water. 133 rows although all compounds have a characteristic solubility in water at. Magnesium Insoluble Water.
From www.numerade.com
SOLVED Which of the following compounds is INSOLUBLE in water? Select one magnesium phosphate Magnesium Insoluble Water Find the molar solubility of a compound in water with the solubility calculator, rules or a table. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Magnesium hydroxide appears to be insoluble in water. In this section we will apply chemical equilibria to the concept of solubility and introduce. Magnesium Insoluble Water.
From www.youtube.com
Is Mg(OH)2 Soluble or Insoluble in Water? YouTube Magnesium Insoluble Water 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. The hydroxides of. Magnesium Insoluble Water.
From www.sodium-cryolite.com
Water Insoluble Mgf2 Magnesium Powder 7783406 For Optical Coating Magnesium Insoluble Water 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Water solubility of magnesium hydroxide is 12 mg/l. However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium. Magnesium Insoluble Water.
From stock.adobe.com
The zeolite softening process is used for removing both the temporary and permanent hardness of Magnesium Insoluble Water The hydroxides of transition metals and. Water solubility of magnesium hydroxide is 12 mg/l. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Other magnesium compounds. Magnesium Insoluble Water.
From www.bwtshop.co.uk
Magnesium Mineralized Water BWT Individualised Drinking Water Magnesium Insoluble Water The hydroxides of transition metals and. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. However, if it is shaken in water and filtered, the solution is slightly basic. Magnesium hydroxide appears to be insoluble in water. Find the molar solubility of a compound in water with the solubility. Magnesium Insoluble Water.
From odanlab.com
MAGNESIUMODAN Odan Laboratories Ltd. Magnesium Insoluble Water However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of transition metals and. Other magnesium compounds are clearly more water soluble, for example magnesium. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Magnesium hydroxide appears to be insoluble in water.. Magnesium Insoluble Water.
From www.pinterest.com
Making magnesium carbonate the formation of an insoluble salt in water Learn Chemistry Magnesium Insoluble Water Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of transition metals and. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. Water solubility of magnesium hydroxide is 12 mg/l. However, if it is shaken in water and filtered, the solution is slightly basic. Magnesium hydroxide appears to. Magnesium Insoluble Water.
From www.amazon.co.uk
Natural Magnesium Water 500mg Magnesium in 500ml ''Donat Mg'' Bulk Pack 12x0,5L The Ultimate Magnesium Insoluble Water Magnesium hydroxide appears to be insoluble in water. Other magnesium compounds are clearly more water soluble, for example magnesium. However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Find the molar solubility. Magnesium Insoluble Water.
From drdavisinfinitehealth.com
Magnesium Water StepbyStep Dr. William Davis Magnesium Insoluble Water The hydroxides of transition metals and. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. However, if it is shaken in water and filtered, the solution is slightly basic. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Other magnesium. Magnesium Insoluble Water.
From brainly.in
What happens when Magnesium oxide is dissolved in water? Write a word equation for this process Magnesium Insoluble Water The hydroxides of transition metals and. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. In this section we will apply chemical equilibria to the concept of solubility and introduce a type. Magnesium Insoluble Water.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Magnesium Insoluble Water The hydroxides of transition metals and. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some. Magnesium Insoluble Water.
From theheartysoul.com
Magnesium Bicarbonate Water Recipe for Headaches and Muscle Pain The Hearty Soul Magnesium Insoluble Water In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The hydroxides of transition metals and. Find the molar solubility of a compound in water with the solubility calculator,. Magnesium Insoluble Water.
From betteryou.com
Magnesium Water Hydrate BetterYou Magnesium Insoluble Water The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Water solubility of magnesium hydroxide is 12 mg/l. Magnesium hydroxide appears to be insoluble in water. The hydroxides of transition metals and. 133 rows although all compounds have a characteristic solubility in water at a given temperature,. Magnesium Insoluble Water.
From fineartamerica.com
Magnesium Reacting With Water Photograph by Andrew Lambert Photography Magnesium Insoluble Water Magnesium hydroxide appears to be insoluble in water. Other magnesium compounds are clearly more water soluble, for example magnesium. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. 133 rows although. Magnesium Insoluble Water.
From www.pnnl.gov
Simple Process Extracts Valuable Magnesium Salt from Seawater Feature PNNL Magnesium Insoluble Water Water solubility of magnesium hydroxide is 12 mg/l. Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of. Magnesium Insoluble Water.
From www.boundtree.com
Magnesium Sulfate in Water, 40mg/mL, 100mL Bag Bound Tree Magnesium Insoluble Water The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Water solubility of magnesium hydroxide is 12 mg/l. The hydroxides of transition metals and. Magnesium hydroxide appears to be insoluble in water. Other magnesium compounds are clearly more water soluble, for example magnesium. 133 rows although all. Magnesium Insoluble Water.
From www.sodium-cryolite.com
Water Insoluble Mgf2 Magnesium Powder 7783406 For Optical Coating Magnesium Insoluble Water Magnesium hydroxide appears to be insoluble in water. However, if it is shaken in water and filtered, the solution is slightly basic. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of transition metals and.. Magnesium Insoluble Water.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Insoluble Water In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Water solubility of magnesium hydroxide is 12 mg/l. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Magnesium hydroxide appears to be insoluble in water.. Magnesium Insoluble Water.
From edu.rsc.org
Making magnesium carbonate the formation of an insoluble salt in water Experiment RSC Education Magnesium Insoluble Water The hydroxides of transition metals and. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Magnesium hydroxide appears to be insoluble in water. 133 rows. Magnesium Insoluble Water.
From www.youtube.com
Is MgCl2 Soluble or Insoluble in Water? YouTube Magnesium Insoluble Water In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Water solubility of magnesium hydroxide is 12 mg/l. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Other magnesium compounds are clearly more water soluble, for example magnesium.. Magnesium Insoluble Water.
From firewaterstar.com
Magnesium Liquid Drops FireWaterStar Magnesium Insoluble Water The hydroxides of transition metals and. Magnesium hydroxide appears to be insoluble in water. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. However, if it is shaken in water and filtered, the solution is slightly basic. In this section we will apply chemical equilibria to the concept of. Magnesium Insoluble Water.
From www.standard-chem.com
What is the white insoluble matter in the aqueous solution of magnesium chloride? Magnesium Insoluble Water The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Other magnesium compounds are clearly more water soluble, for example magnesium. Water solubility of magnesium hydroxide is 12 mg/l. Magnesium hydroxide appears to be insoluble in water. The hydroxides of transition metals and. In this section we. Magnesium Insoluble Water.
From betteryou.com
Magnesium Water Hydrate BetterYou Magnesium Insoluble Water In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. However, if it is shaken in water and filtered, the solution is slightly basic. 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. The hydroxides of transition metals. Magnesium Insoluble Water.
From www.bwt.com
Magnesium mineralized water BWT Magnesium Insoluble Water Other magnesium compounds are clearly more water soluble, for example magnesium. However, if it is shaken in water and filtered, the solution is slightly basic. Water solubility of magnesium hydroxide is 12 mg/l. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. The hydroxides of transition metals and.. Magnesium Insoluble Water.
From mammothmemory.net
Magnesium reacts with water but not vigorously Magnesium Insoluble Water However, if it is shaken in water and filtered, the solution is slightly basic. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. Water solubility of magnesium hydroxide is 12 mg/l. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are. Magnesium Insoluble Water.
From holisticacupuncture.com.au
Magnesium Bath Flakes 750g Magnesium Insoluble Water 133 rows although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are. Water solubility of magnesium hydroxide is 12 mg/l. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Find the molar solubility of a compound in water. Magnesium Insoluble Water.
From drdavisinfinitehealth.com
Magnesium Water StepbyStep Dr. William Davis Magnesium Insoluble Water The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. However, if it is shaken in water and filtered, the solution is slightly basic. Find the molar solubility of a compound in water with the solubility calculator, rules or a table. Magnesium hydroxide appears to be insoluble. Magnesium Insoluble Water.
From www.bwt.com
Magnesium Mineralized Water 12 Pack BWT Magnesium Insoluble Water Magnesium hydroxide appears to be insoluble in water. Water solubility of magnesium hydroxide is 12 mg/l. However, if it is shaken in water and filtered, the solution is slightly basic. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. 133 rows although all compounds have a. Magnesium Insoluble Water.
From www.bulkreefsupply.com
Magnesium Dry Aquaforest Bulk Reef Supply Magnesium Insoluble Water Find the molar solubility of a compound in water with the solubility calculator, rules or a table. Other magnesium compounds are clearly more water soluble, for example magnesium. The hydroxides of transition metals and. The hydroxides of alkaline earth metals, like magnesium (mg 2+), calcium (ca 2+), strontium (sr 2+), and barium (ba 2+), are mildly soluble. Water solubility of. Magnesium Insoluble Water.