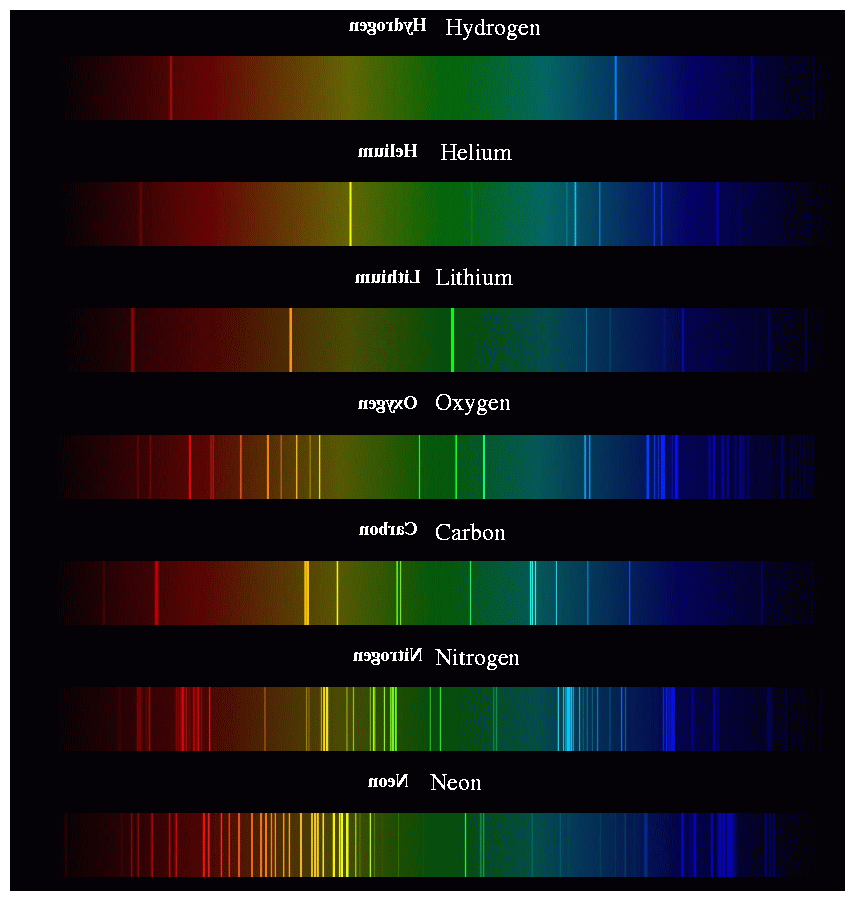
Emission Spectra For Lithium . 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed. The three atomic emission spectra for lithium can be shown on graph bellow: Vacuum wavelength (å) spectrum : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited.
from spiff.rit.edu
When hydrogen gas is placed. The three atomic emission spectra for lithium can be shown on graph bellow: 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Vacuum wavelength (å) spectrum : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
Spectrographs and Spectra
Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for lithium can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed. Vacuum wavelength (å) spectrum :
From www.researchgate.net
Emission spectra of (a) Li + = 0, (b) Li + = 5 and (c) Li + = 10 at Emission Spectra For Lithium Vacuum wavelength (å) spectrum : 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra For Lithium.
From www.researchgate.net
Leaching of Li + in water. (a) The emission spectrum of Li + in water Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Vacuum wavelength (å) spectrum : Emission and absorption spectra. Emission Spectra For Lithium.
From www.slideserve.com
PPT Atomic Emission Spectra PowerPoint Presentation, free download Emission Spectra For Lithium When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The three atomic emission spectra for lithium can be shown on graph bellow: Vacuum wavelength (å) spectrum : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the. Emission Spectra For Lithium.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The emission spectrum (or line spectrum) of. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectrum of plasma radiation in PLM device with the lithium Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about. Emission Spectra For Lithium.
From chart-studio.plotly.com
Lithium Ion Emission Spectrum line chart made by Steiner.zion plotly Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : Vacuum wavelength (å) spectrum : When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Emission Spectra For Lithium.
From www.researchgate.net
Resonant C Kemission spectra of model compounds, (A) lithium Emission Spectra For Lithium Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra For Lithium.
From www.scribd.com
LAB5 Getting To Know Some Elements Students Report PDF Emission Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The three atomic emission spectra for lithium can be shown on graph bellow: The emission spectrum (or line spectrum) of a chemical element is the unique pattern. Emission Spectra For Lithium.
From www.researchgate.net
Enlargement of the emission spectrum of Li 3.5 (Y 0.9 Eu 0.1 ) 1.5 (MoO Emission Spectra For Lithium Vacuum wavelength (å) spectrum : 72 rows lithium (li) strong lines of lithium ( li ) intensity : When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectrum of the Lymanline of a lithium LPP spectrum in a Emission Spectra For Lithium Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. 72 rows lithium (li) strong lines of lithium ( li ) intensity : The three atomic emission spectra for lithium can be shown on graph bellow: When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of. Emission Spectra For Lithium.
From www.researchgate.net
Emission and excitation spectra of Li 3 Ba 2 Eu 3 (MoO 4 ) 8 at 3 K Emission Spectra For Lithium The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 72 rows lithium (li) strong lines of lithium ( li ) intensity : When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by. Emission Spectra For Lithium.
From www.researchgate.net
The excitation (a) and emission (b) spectra of Li 3 BaSrGd 3 (MoO 4 ) 8 Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by. Emission Spectra For Lithium.
From www.researchgate.net
LIBS plasma emission spectrum of a Li layer analyzed in atmospheric air Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Vacuum wavelength (å) spectrum : The three atomic emission spectra for lithium can be shown on graph bellow: 72 rows lithium (li) strong lines of lithium ( li ) intensity : When hydrogen gas is placed. The emission spectrum (or. Emission Spectra For Lithium.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for lithium can be shown on graph bellow: Vacuum wavelength (å) spectrum : When hydrogen gas. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectrum of the Lymanline of a lithium LPP spectrum in a Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy,. Emission Spectra For Lithium.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra For Lithium When hydrogen gas is placed. The three atomic emission spectra for lithium can be shown on graph bellow: 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Emission spectrum lithium refers to the specific pattern of. Emission Spectra For Lithium.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra For Lithium The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Emission. Emission Spectra For Lithium.
From www.researchgate.net
The emission spectrum of Lithium Fluoride (í µí°¿í µí± í µí°¹) using í Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: 72 rows lithium (li) strong lines of lithium ( li ) intensity : Vacuum wavelength (å) spectrum : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Emission spectrum lithium refers to the specific pattern of. Emission Spectra For Lithium.
From www.researchgate.net
Xray induced emission spectra of Li 6 Gd(BO 3 ) 3 phosphor with Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: When hydrogen gas is placed. 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Vacuum wavelength (å) spectrum : Emission spectrum lithium refers. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectra of Li* He n at various temperatures in Download Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Vacuum wavelength (å) spectrum : 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectrum of the Lymanline of a lithium LPP spectrum in a Emission Spectra For Lithium Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed. Vacuum wavelength (å) spectrum : The three atomic emission spectra for lithium can be shown on graph bellow: Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium. Emission Spectra For Lithium.
From mohamednewscaldwell.blogspot.com
Determination of Sodium by Flame Emission Spectroscopy Emission Spectra For Lithium The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 72 rows lithium (li) strong lines of lithium ( li ) intensity : When hydrogen gas is placed. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by. Emission Spectra For Lithium.
From www.researchgate.net
Mössbauer emission spectra of 67 Cu( 67 Zn) in lithium halides relative Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 72 rows lithium (li) strong lines of lithium ( li ) intensity : Vacuum. Emission Spectra For Lithium.
From www.researchgate.net
Left The emission spectra for water and different NaCl concentration Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of. Emission Spectra For Lithium.
From www.shutterstock.com
Absorption Emission Spectrum Lithium Stock Vector (Royalty Free Emission Spectra For Lithium The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Vacuum wavelength (å) spectrum : The three atomic emission spectra for lithium can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses. Emission Spectra For Lithium.
From scisyn.com
Spectra Examples Emission Spectra For Lithium When hydrogen gas is placed. 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Vacuum wavelength (å) spectrum : The three atomic emission spectra for lithium can be shown on. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectra of pure and Ag doped lithium lanthanum titanate (LLTO Emission Spectra For Lithium When hydrogen gas is placed. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. The three atomic emission spectra for lithium can be shown on graph bellow: 72 rows lithium (li) strong lines of lithium ( li ) intensity : The emission spectrum (or line spectrum) of a chemical. Emission Spectra For Lithium.
From www.researchgate.net
The spectrum of lithium atom line emission. Download Table Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The three atomic emission spectra for lithium can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. 72 rows lithium (li) strong lines of. Emission Spectra For Lithium.
From architecturalstudio.com
Lithium Emission Spectrum Emission Spectra For Lithium The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for lithium can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When. Emission Spectra For Lithium.
From www.researchgate.net
(PDF) Quantifying lithium concentration gradients in the graphite Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra For Lithium.
From www.researchgate.net
Leaching of Li + in water. (a) The emission spectrum of Li + in water Emission Spectra For Lithium When hydrogen gas is placed. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. Vacuum wavelength (å) spectrum : The three atomic emission spectra for lithium can be shown. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectrum of Li 2 CaSiO 4 xPr 3+ phosphor with excitation Emission Spectra For Lithium When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The three atomic emission spectra for lithium can be shown on graph bellow: Vacuum wavelength (å) spectrum : Emission spectrum lithium refers to the specific pattern of wavelengths or. Emission Spectra For Lithium.
From www.semanticscholar.org
Raman Spectroscopy for LiNi1/3Mn1/3Co1/3O2 Composite Positive Emission Spectra For Lithium The three atomic emission spectra for lithium can be shown on graph bellow: Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the. Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. 72 rows lithium (li) strong lines of. Emission Spectra For Lithium.
From www.researchgate.net
Emission and excitation spectra of Li 3 Ba 2 Eu 3 (MoO 4 ) 8 at 3 K Emission Spectra For Lithium Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Emission and absorption spectra form the basis of spectroscopy,. Emission Spectra For Lithium.
From www.researchgate.net
Emission spectra of different concentrations of Sm 3+ doped strontium Emission Spectra For Lithium 72 rows lithium (li) strong lines of lithium ( li ) intensity : Emission spectrum lithium refers to the specific pattern of wavelengths or colors of light emitted by lithium atoms when excited. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission. Emission Spectra For Lithium.