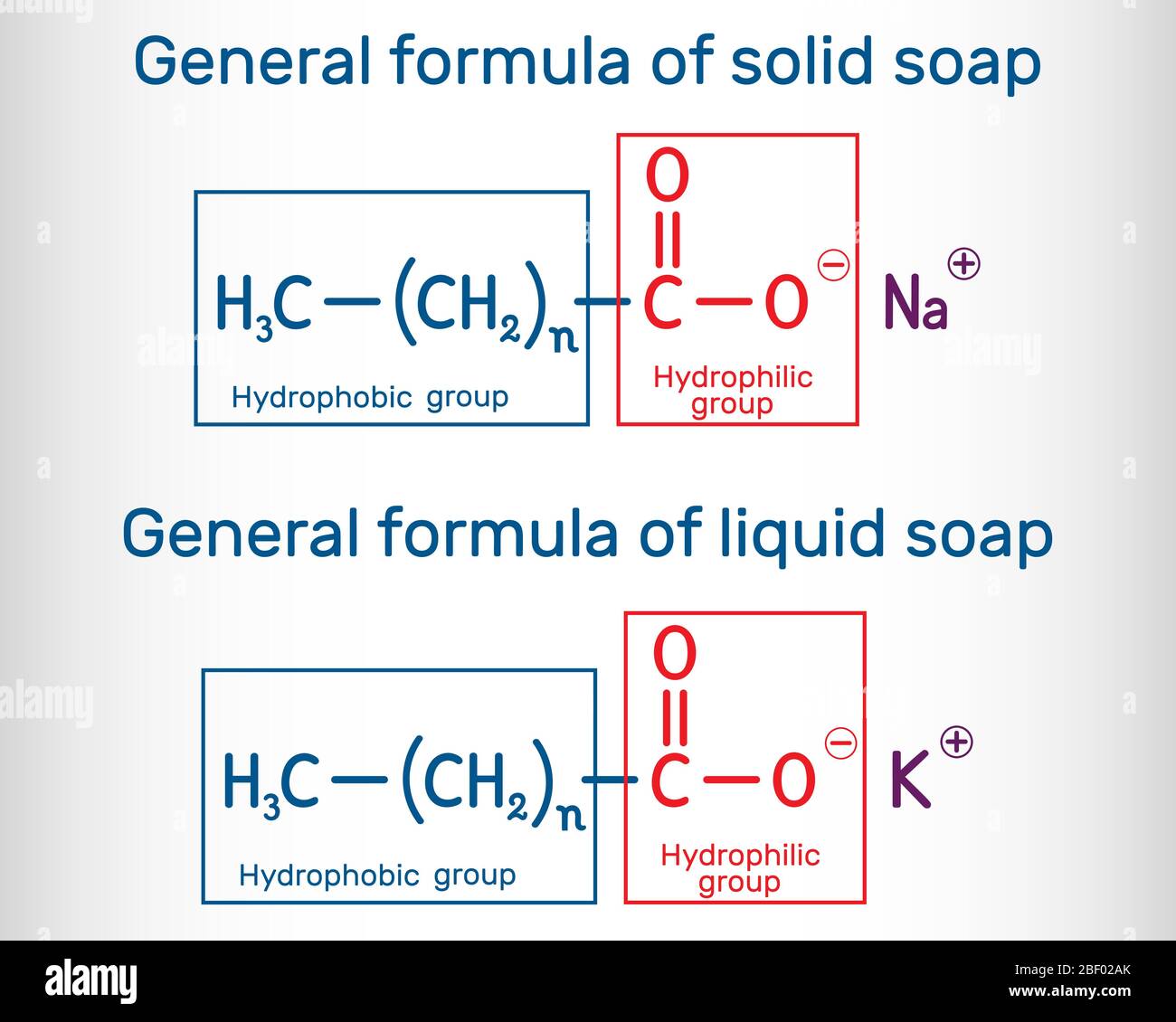
What Are Soaps In Chemistry . Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The oldest amphiphilic cleaning agent known to humans is soap. It is the result of a. The reaction produces sodium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids.
from www.alamy.com
It is the result of a. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The oldest amphiphilic cleaning agent known to humans is soap. The reaction produces sodium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification.
General formula of solid and liquid soap molecule. RCOONa, RCOOK
What Are Soaps In Chemistry It is the result of a. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The oldest amphiphilic cleaning agent known to humans is soap. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. It is the result of a. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The reaction produces sodium salts of.
From cbse.myindialist.com
Chemistry X Carbon and its Compounds SOAPS AND DETERGENTS CBSE What Are Soaps In Chemistry The oldest amphiphilic cleaning agent known to humans is soap. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long hydrocarbon chain, sometimes. What Are Soaps In Chemistry.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting. What Are Soaps In Chemistry.
From cosmosmagazine.com
The chemistry of soap What Are Soaps In Chemistry Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The oldest amphiphilic cleaning agent known to humans is soap. Soap is a classic cleaning agent that. What Are Soaps In Chemistry.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK What Are Soaps In Chemistry Soap is a classic cleaning agent that has been used for centuries. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat. What Are Soaps In Chemistry.
From www.youtube.com
Chemistry 101 How does soap work? YouTube What Are Soaps In Chemistry Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The oldest amphiphilic cleaning agent known to humans is soap. It is the result of a.. What Are Soaps In Chemistry.
From thesoapmoleculeco.com
The Soap Molecule Co. What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The reaction produces sodium salts of. It is the result of a. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The oldest amphiphilic cleaning agent known to humans. What Are Soaps In Chemistry.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis What Are Soaps In Chemistry Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. It is the result of a. Soap is a classic cleaning agent that has been used for centuries. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are sodium or. What Are Soaps In Chemistry.
From www.pinterest.es
Hand washing with soap vector illustration. Educational explanation What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The reaction produces sodium salts of. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves. What Are Soaps In Chemistry.
From cen.acs.org
Periodic graphics Soap versus body wash What Are Soaps In Chemistry It is the result of a. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance,. What Are Soaps In Chemistry.
From www.researchgate.net
1. Composition of bar of soap and liquid soap Download Table What Are Soaps In Chemistry The reaction produces sodium salts of. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. It is the result of a. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long hydrocarbon. What Are Soaps In Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID What Are Soaps In Chemistry It is the result of a. The oldest amphiphilic cleaning agent known to humans is soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification.. What Are Soaps In Chemistry.
From ar.inspiredpencil.com
Soap Molecule Structure What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps are sodium or potassium fatty acids salts, produced from the. What Are Soaps In Chemistry.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents What Are Soaps In Chemistry Soap is a classic cleaning agent that has been used for centuries. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The oldest amphiphilic cleaning agent known to humans is soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known. What Are Soaps In Chemistry.
From ar.inspiredpencil.com
Soap Molecule Polar Or Nonpolar What Are Soaps In Chemistry It is the result of a. The reaction produces sodium salts of. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soap is a classic cleaning agent that has been used for centuries. Soaps are cleaning agents that are usually made by reacting alkali (e.g.,. What Are Soaps In Chemistry.
From www.youtube.com
Types of Soap, Chemistry Lecture Sabaq.pk YouTube What Are Soaps In Chemistry The oldest amphiphilic cleaning agent known to humans is soap. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. It is the result. What Are Soaps In Chemistry.
From www.slideshare.net
Chemistry of soaps What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. It is the result of a. Soap is a classic cleaning agent that has been used for centuries. Soap is a salt of. What Are Soaps In Chemistry.
From www.shutterstock.com
Soap Chemistry Soap Chemistry Formula Structure Stock Vector (Royalty What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known. What Are Soaps In Chemistry.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab What Are Soaps In Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The oldest amphiphilic cleaning agent known to humans is soap. Soap is a classic cleaning agent that has been used for centuries. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats. What Are Soaps In Chemistry.
From slideshare.net
Soap and Detergents What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are cleaning agents that are usually made. What Are Soaps In Chemistry.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap What Are Soaps In Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide). What Are Soaps In Chemistry.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences What Are Soaps In Chemistry The reaction produces sodium salts of. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Its fundamental chemistry involves the combination of fats or oils with an alkaline. What Are Soaps In Chemistry.
From www.goodreads.com
Soap Chemistry Discover The Basics Of Soap Chemistry by Wan Yamane What Are Soaps In Chemistry Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in. What Are Soaps In Chemistry.
From www.slideshare.net
Chemistry of soaps What Are Soaps In Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The reaction produces sodium salts of. Soap is a salt of an alkali metal, such as. What Are Soaps In Chemistry.
From www.pinterest.com
How Soap Work? Soap, Cleanse, Basic concepts What Are Soaps In Chemistry It is the result of a. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Its fundamental chemistry involves the combination of fats or oils with. What Are Soaps In Chemistry.
From busy.org
Saponification 1/2 What Are Soaps In Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Its fundamental chemistry involves the combination of fats or oils. What Are Soaps In Chemistry.
From www.pinterest.com
Cleansing Action Of Soap. Soap, Cleanse, Molecules What Are Soaps In Chemistry It is the result of a. Soap is a classic cleaning agent that has been used for centuries. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. The oldest amphiphilic cleaning agent known to humans is soap. Its fundamental chemistry involves the combination of fats or oils. What Are Soaps In Chemistry.
From www.pinterest.com
An Introduction to Chemistry Soap, Soap making, Chemical reactions What Are Soaps In Chemistry The oldest amphiphilic cleaning agent known to humans is soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soaps are cleaning agents that are. What Are Soaps In Chemistry.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project What Are Soaps In Chemistry Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. The reaction produces sodium salts of. The oldest amphiphilic cleaning agent known to humans is soap. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known. What Are Soaps In Chemistry.
From www.thoughtco.com
How Saponification Makes Soap What Are Soaps In Chemistry The oldest amphiphilic cleaning agent known to humans is soap. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. It is the result. What Are Soaps In Chemistry.
From www.slideshare.net
Chemistry of soaps What Are Soaps In Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification.. What Are Soaps In Chemistry.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry What Are Soaps In Chemistry Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. Soap is a classic cleaning agent that has been used for centuries. The reaction produces sodium salts of. Its. What Are Soaps In Chemistry.
From easychem.com.au
The Difference Between Soaps And Synthetic Detergents EasyChem Australia What Are Soaps In Chemistry It is the result of a. Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soap is a classic cleaning agent that has been used for centuries. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are sodium or. What Are Soaps In Chemistry.
From ar.inspiredpencil.com
Soap Molecular Formula What Are Soaps In Chemistry Soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The oldest amphiphilic cleaning agent known to humans is soap. It is the result of a. Its fundamental. What Are Soaps In Chemistry.
From www.slideshare.net
Chemistry of soaps What Are Soaps In Chemistry Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat or fatty acids. The reaction produces sodium salts of. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are sodium or potassium fatty acids. What Are Soaps In Chemistry.
From www.thoughtco.com
How Soap Works What Are Soaps In Chemistry The reaction produces sodium salts of. The oldest amphiphilic cleaning agent known to humans is soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. It is the result of a. Soaps are cleaning agents that are usually made by reacting alkali (e.g., sodium hydroxide) with naturally occurring fat. What Are Soaps In Chemistry.