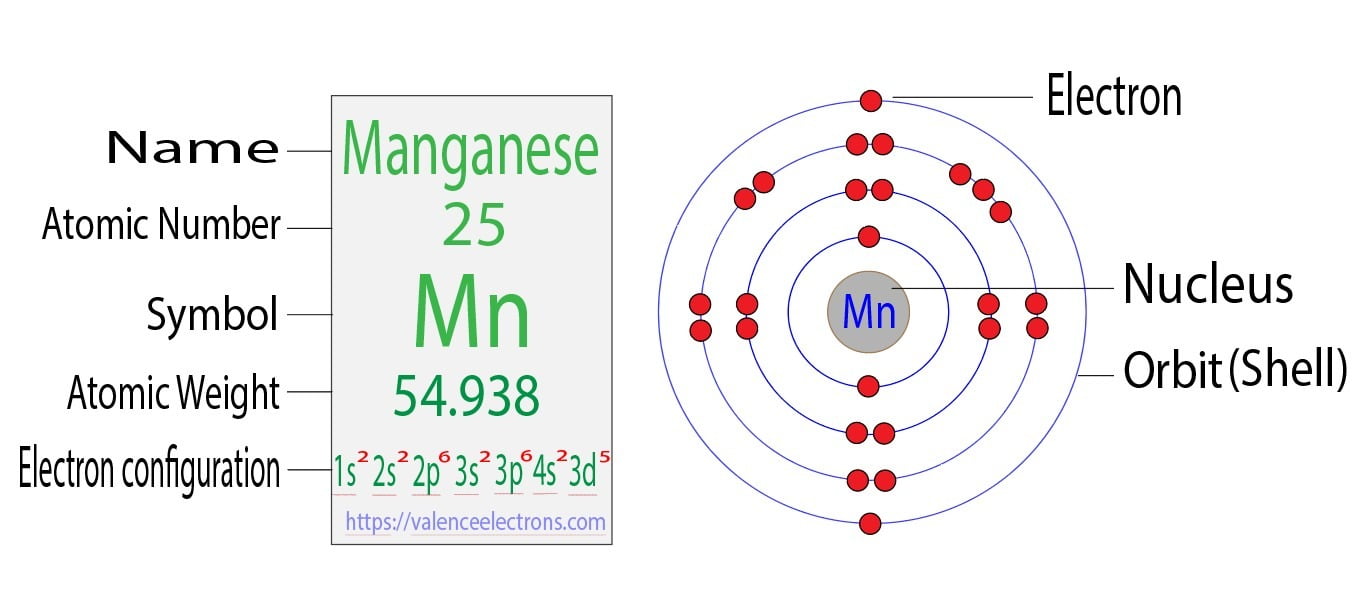
Electron Configuration Of Magnesium Ion Mg2+ . There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). The electronic configuration of a magnesium ion can. The correct option is a 1s2 2s2 2p6. Krypton, neon, xenon, argon, and helium. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Atomic number of magnesium is 12. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. Magnesium atom loses 2 electrons from its outermost shell to. In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not similar to ar.
from valenceelectrons.com
Magnesium atom loses 2 electrons from its outermost shell to. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Krypton, neon, xenon, argon, and helium. In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not similar to ar. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Atomic number of magnesium is 12. The electronic configuration of a magnesium ion can. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas:
Electron Configuration for Magnesium and ion(Mg2+)
Electron Configuration Of Magnesium Ion Mg2+ Atomic number of magnesium is 12. Magnesium atom loses 2 electrons from its outermost shell to. In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electronic configuration of a magnesium ion can. It is not similar to ar. Atomic number of magnesium is 12. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: The correct option is a 1s2 2s2 2p6. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). Krypton, neon, xenon, argon, and helium. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+.
From www.newtondesk.com
magnesium electron configuration Newton Desk Electron Configuration Of Magnesium Ion Mg2+ The correct option is a 1s2 2s2 2p6. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). The electronic configuration. Electron Configuration Of Magnesium Ion Mg2+.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Electron Configuration Of Magnesium Ion Mg2+ Magnesium atom loses 2 electrons from its outermost shell to. The electronic configuration of a magnesium ion can. Krypton, neon, xenon, argon, and helium. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). The electron configuration of the magnesium ion mg2+ is similar. Electron Configuration Of Magnesium Ion Mg2+.
From schematicellsclours.z21.web.core.windows.net
Magnesium Electron Dot Diagram Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas:. Electron Configuration Of Magnesium Ion Mg2+.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Electron Configuration Of Magnesium Ion Mg2+ For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). The electronic configuration of a magnesium ion can. The correct option is a 1s2 2s2 2p6. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble. Electron Configuration Of Magnesium Ion Mg2+.
From valenceelectrons.com
Electron Configuration for Magnesium (Mg, Mg2+ ion) Electron Configuration Of Magnesium Ion Mg2+ There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: It is not similar to ar. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). As a result of losing its two valence electrons,. Electron Configuration Of Magnesium Ion Mg2+.
From brainly.in
Write lewis dot structure of Mg2+ Brainly.in Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Atomic number of magnesium is 12. Krypton, neon, xenon, argon, and helium. It is not similar to ar. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by. Electron Configuration Of Magnesium Ion Mg2+.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Electron Configuration Of Magnesium Ion Mg2+ The correct option is a 1s2 2s2 2p6. It is not similar to ar. Atomic number of magnesium is 12. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. The electronic configuration of a magnesium. Electron Configuration Of Magnesium Ion Mg2+.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Electron Configuration Of Magnesium Ion Mg2+ The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by. Electron Configuration Of Magnesium Ion Mg2+.
From valenceelectrons.com
Electron Configuration for Magnesium (Mg, Mg2+ ion) Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Atomic number of magnesium is 12. The correct option is a 1s2 2s2 2p6. In this video we will write the electron configuration for mg 2+, the. Electron Configuration Of Magnesium Ion Mg2+.
From socratic.org
What is the electronic configuration of Mg^(2+) ion? Socratic Electron Configuration Of Magnesium Ion Mg2+ For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: It is not similar to ar. As a result of losing its two valence electrons,. Electron Configuration Of Magnesium Ion Mg2+.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Electron Configuration Of Magnesium Ion Mg2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. As a result of losing its two. Electron Configuration Of Magnesium Ion Mg2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. It is not similar to ar. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas. Electron Configuration Of Magnesium Ion Mg2+.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Electron Configuration Of Magnesium Ion Mg2+ It is not similar to ar. Atomic number of magnesium is 12. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The correct option is a 1s2 2s2 2p6. There are five options, each stating that the mg2+ ion has the electron configuration of a different. Electron Configuration Of Magnesium Ion Mg2+.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Electron Configuration Of Magnesium Ion Mg2+ Magnesium atom loses 2 electrons from its outermost shell to. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Krypton, neon, xenon, argon, and helium. As a result of losing its. Electron Configuration Of Magnesium Ion Mg2+.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Magnesium atom loses 2 electrons from its outermost shell to. For mg2+, which is a cation with a. Electron Configuration Of Magnesium Ion Mg2+.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Electron Configuration Of Magnesium Ion Mg2+ It is not similar to ar. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). As a result of losing its two valence electrons, the magnesium ion. Electron Configuration Of Magnesium Ion Mg2+.
From mungfali.com
Magnesium Ion Electron Configuration Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Magnesium atom loses 2 electrons from its outermost shell to. The electronic configuration of a magnesium ion can. The correct option is a 1s2 2s2 2p6. Atomic. Electron Configuration Of Magnesium Ion Mg2+.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electronic configuration of a magnesium ion can. Krypton, neon, xenon, argon, and helium. It is not similar to. Electron Configuration Of Magnesium Ion Mg2+.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium Ion Mg2+ Magnesium atom loses 2 electrons from its outermost shell to. The electronic configuration of a magnesium ion can. The correct option is a 1s2 2s2 2p6. Krypton, neon, xenon, argon, and helium. It is not similar to ar. In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of mg2+ is 1s². Electron Configuration Of Magnesium Ion Mg2+.
From mavink.com
Draw The Orbital Diagram For Magnesium Electron Configuration Of Magnesium Ion Mg2+ As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. In this video we will write the electron configuration for mg 2+, the magnesium ion. Krypton, neon, xenon, argon, and helium. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas. Electron Configuration Of Magnesium Ion Mg2+.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium Ion Mg2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: Atomic number of magnesium is 12. Krypton, neon, xenon, argon, and helium. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and. Electron Configuration Of Magnesium Ion Mg2+.
From www.chegg.com
Solved Write the electron configuration for Mg2+ ion. Select Electron Configuration Of Magnesium Ion Mg2+ Atomic number of magnesium is 12. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: Krypton, neon, xenon, argon, and helium. The electronic configuration of a magnesium ion can. In this video we will write the electron configuration for mg 2+, the magnesium ion. The correct option is a 1s2. Electron Configuration Of Magnesium Ion Mg2+.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Electron Configuration Of Magnesium Ion Mg2+ It is not similar to ar. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. In this video we will write the electron configuration for mg 2+, the magnesium ion. The correct option is a 1s2 2s2 2p6. Atomic number of magnesium is 12. The electronic configuration of a magnesium ion can. The electron. Electron Configuration Of Magnesium Ion Mg2+.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not similar to ar. As a result of losing its two valence. Electron Configuration Of Magnesium Ion Mg2+.
From www.numerade.com
SOLVED 38. What ion is likely to be formed by magnesium (Mg) in Electron Configuration Of Magnesium Ion Mg2+ The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. In this video we will write the electron configuration for mg 2+, the magnesium ion. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. Krypton, neon, xenon, argon, and helium. For mg2+, which is a cation. Electron Configuration Of Magnesium Ion Mg2+.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. Atomic number of magnesium is 12. In this. Electron Configuration Of Magnesium Ion Mg2+.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium Ion Mg2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. Krypton, neon, xenon, argon, and helium. For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). The correct option is a 1s2 2s2 2p6. As a result of losing. Electron Configuration Of Magnesium Ion Mg2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as. Electron Configuration Of Magnesium Ion Mg2+.
From ar.inspiredpencil.com
Magnesium Atom Structure Electron Configuration Of Magnesium Ion Mg2+ Atomic number of magnesium is 12. The correct option is a 1s2 2s2 2p6. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium atom loses 2 electrons from its outermost. Electron Configuration Of Magnesium Ion Mg2+.
From liondigitalartillustrations.blogspot.com
draw the lewis dot structure for mg2+ liondigitalartillustrations Electron Configuration Of Magnesium Ion Mg2+ Atomic number of magnesium is 12. Magnesium atom loses 2 electrons from its outermost shell to. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: In this video we will write the electron configuration for. Electron Configuration Of Magnesium Ion Mg2+.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog Electron Configuration Of Magnesium Ion Mg2+ The electronic configuration of a magnesium ion can. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. Magnesium atom loses 2 electrons from its outermost shell to. Krypton, neon, xenon, argon, and helium. There are five options, each stating that the mg2+ ion has the electron configuration of a different noble. Electron Configuration Of Magnesium Ion Mg2+.
From www.youtube.com
How to find the Oxidation Number for the Mg2+ ion. (Magnesium ion Electron Configuration Of Magnesium Ion Mg2+ The correct option is a 1s2 2s2 2p6. The electronic configuration of a magnesium ion can. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. It is not similar to ar. Magnesium atom loses 2 electrons from its outermost shell to. In this video we will write the electron configuration for mg 2+, the. Electron Configuration Of Magnesium Ion Mg2+.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium Ion Mg2+ The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). For mg2+, which is a cation with a positive charge of +2, the electron configuration can be determined by considering the neutral atom of magnesium (mg). Atomic number of magnesium is 12. The correct option is a. Electron Configuration Of Magnesium Ion Mg2+.
From guidemanualeruptivity.z14.web.core.windows.net
Electronic Structure Diagram For Magnesium Electron Configuration Of Magnesium Ion Mg2+ Krypton, neon, xenon, argon, and helium. Magnesium atom loses 2 electrons from its outermost shell to. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that. Electron Configuration Of Magnesium Ion Mg2+.
From www.numerade.com
SOLVED Write the full electronic configurations of both Mg and Mg2 Electron Configuration Of Magnesium Ion Mg2+ There are five options, each stating that the mg2+ ion has the electron configuration of a different noble gas: Magnesium atom loses 2 electrons from its outermost shell to. The electronic configuration of a magnesium ion can. As a result of losing its two valence electrons, the magnesium ion has a total of ten electrons. The correct option is a. Electron Configuration Of Magnesium Ion Mg2+.