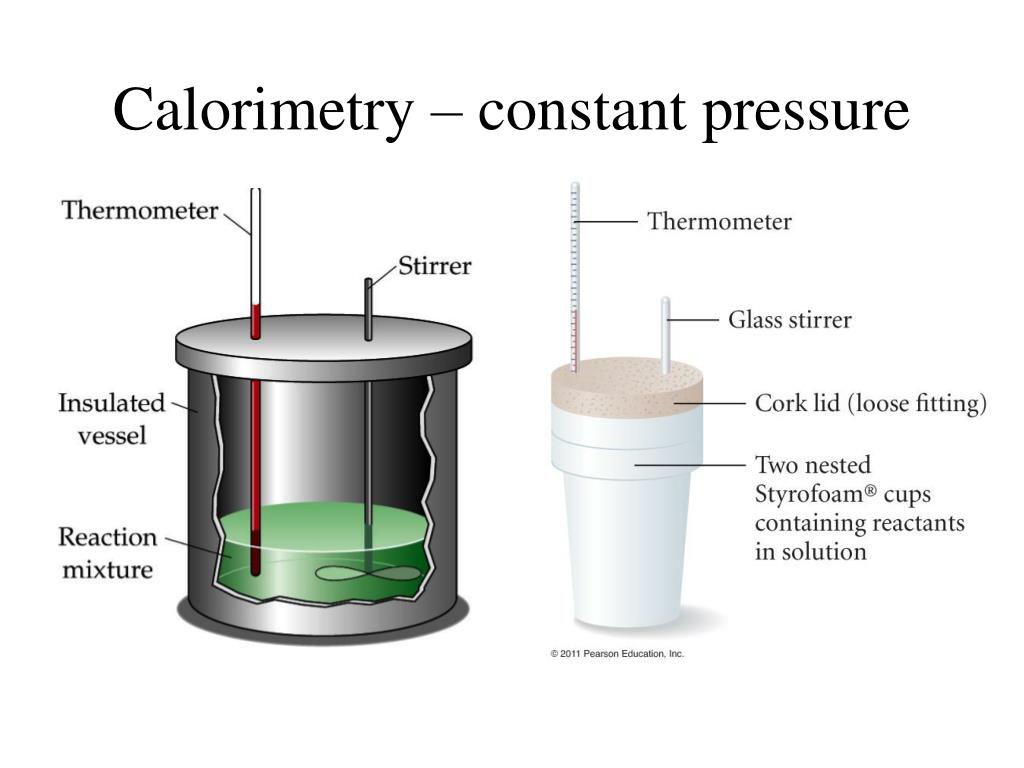
Calorimetry Examples Real Life . Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A bomb calorimeter can calculate the amount of calories in foods we eat. It burns food in a sealed container, and uses the heat from the container to warm surrounding water.
from www.slideserve.com
Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. A bomb calorimeter can calculate the amount of calories in foods we eat.
PPT Thermochemistry PowerPoint Presentation, free download ID2692866
Calorimetry Examples Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A bomb calorimeter can calculate the amount of calories in foods we eat. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Examples Real Life Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A bomb calorimeter can calculate the amount of calories in foods we eat. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. A. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Q = MCΔT Specific Heat & CALORIMETRY PowerPoint Presentation Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A bomb calorimeter can calculate the amount of calories in foods we eat. With a styrofoam calorimeter, the temperature of the substance inside the cup is. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Examples Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Examples Real Life.
From chem.libretexts.org
3.4 Calorimetry Chemistry LibreTexts Calorimetry Examples Real Life Apply the first law of thermodynamics to calorimetry. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the process of measuring. Calorimetry Examples Real Life.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A bomb calorimeter can calculate the amount of calories in foods we. Calorimetry Examples Real Life.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Examples Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Apply the first law of thermodynamics. Calorimetry Examples Real Life.
From giozuzdes.blob.core.windows.net
Examples Of Calorimetry In Real Life at Barbara Bader blog Calorimetry Examples Real Life For example, when an exothermic. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A bomb calorimeter can calculate the amount of calories in foods we eat. Apply the first law of thermodynamics to calorimetry. Compare heat flow from. Calorimetry Examples Real Life.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning Calorimetry Examples Real Life With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s. Calorimetry Examples Real Life.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Examples Real Life A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature. Calorimetry Examples Real Life.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimetry Examples Real Life Apply the first law of thermodynamics to calorimetry. A bomb calorimeter can calculate the amount of calories in foods we eat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry Examples Real Life.
From www.pinterest.com
Calorimeter An object used for measuring the heat of chemical Calorimetry Examples Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A bomb calorimeter can calculate the amount of calories in foods we eat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. With a styrofoam calorimeter, the temperature of the substance. Calorimetry Examples Real Life.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example,. Calorimetry Examples Real Life.
From maestrovirtuale.com
Calorimetria o que você estuda e aplicações Calorimetry Examples Real Life Apply the first law of thermodynamics to calorimetry. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Examples Real Life.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Calorimetry Examples Real Life With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A bomb calorimeter can calculate the. Calorimetry Examples Real Life.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Calorimetry is the process of measuring the amount of heat released or. Calorimetry Examples Real Life.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimetry Examples Real Life A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Compare. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Examples Real Life A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Energy Changes in Reactions PowerPoint Presentation, free Calorimetry Examples Real Life A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate. Calorimetry Examples Real Life.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Examples Real Life A bomb calorimeter can calculate the amount of calories in foods we eat. Apply the first law of thermodynamics to calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. It burns food in a sealed container, and uses the heat from the container to. Calorimetry Examples Real Life.
From 2012books.lardbucket.org
Calorimetry Calorimetry Examples Real Life For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A bomb calorimeter can calculate the amount of calories in foods we eat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Calorimeters & Calorimetry PowerPoint Presentation, free download Calorimetry Examples Real Life A bomb calorimeter can calculate the amount of calories in foods we eat. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Examples Real Life.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Examples Real Life A bomb calorimeter can calculate the amount of calories in foods we eat. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For. Calorimetry Examples Real Life.
From studylib.net
Calorimetry Calorimetry Examples Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device used to measure the amount of heat involved. Calorimetry Examples Real Life.
From www.youtube.com
Calorimetry Example Calculation YouTube Calorimetry Examples Real Life With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Examples Real Life Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its. Calorimetry Examples Real Life.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Examples Real Life A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Calorimetry C ontents Basic Concept Example Whiteboards Bomb Calorimetry Examples Real Life With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s. Calorimetry Examples Real Life.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Apply the first law of thermodynamics to calorimetry. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A bomb calorimeter can. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimetry Examples Real Life For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate. Calorimetry Examples Real Life.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimetry Examples Real Life A bomb calorimeter can calculate the amount of calories in foods we eat. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat. Calorimetry Examples Real Life.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint Calorimetry Examples Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. It burns food in a sealed container, and uses the heat from the container to warm surrounding water. For example, when an exothermic. With a styrofoam calorimeter, the temperature of the substance inside the cup is. Calorimetry Examples Real Life.
From www.youtube.com
Calorimetry examples YouTube Calorimetry Examples Real Life A bomb calorimeter can calculate the amount of calories in foods we eat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. It burns food in a sealed. Calorimetry Examples Real Life.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Examples Real Life Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward,. Calorimetry Examples Real Life.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Examples Real Life It burns food in a sealed container, and uses the heat from the container to warm surrounding water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. With. Calorimetry Examples Real Life.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Examples Real Life With a styrofoam calorimeter, the temperature of the substance inside the cup is measured, a reaction is allowed to take place, and afterward, the temperature is measured a second time. A bomb calorimeter can calculate the amount of calories in foods we eat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features. Calorimetry Examples Real Life.