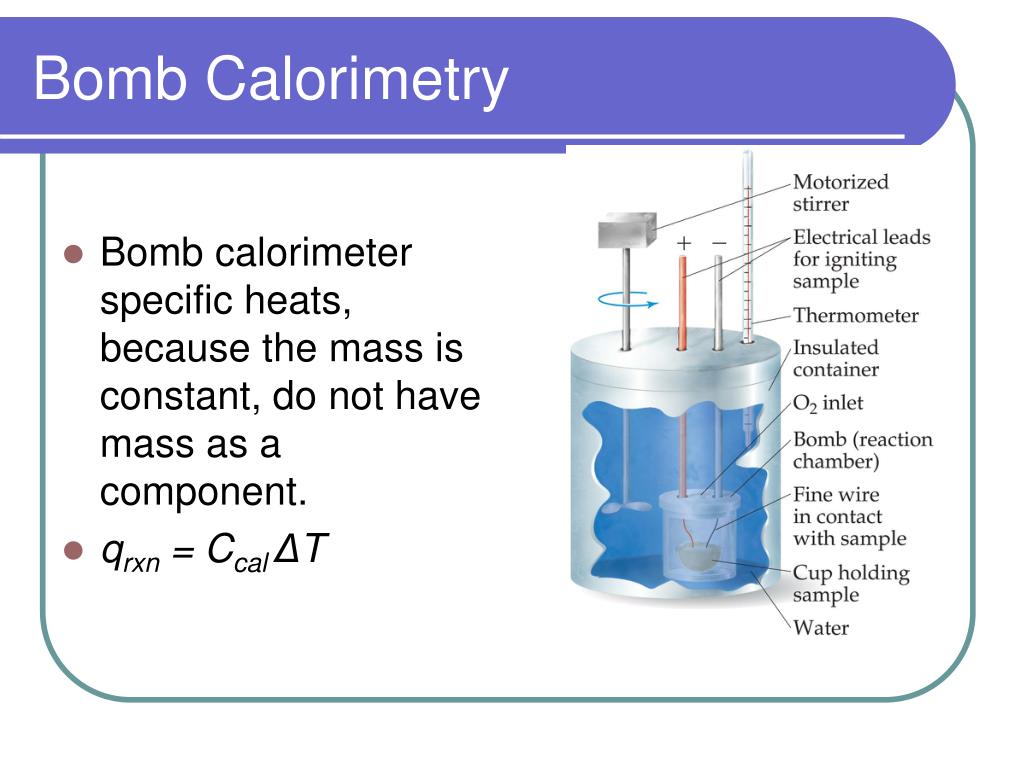
The Calorimeter Equation . Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Calculate the amount of heat released. The heat lost by the warm object is equal to the heat gained by the cooler. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. When heat is absorbed by the solution, q for. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Use the equation for heat transfer \(q = mc\delta t\) to express the. Compare heat flow from hot to cold objects in an ideal. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). To do so, the heat is exchanged with a calibrated object. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry.
from www.slideserve.com
In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Calculate the amount of heat released. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. Use the equation for heat transfer \(q = mc\delta t\) to express the. When heat is absorbed by the solution, q for. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process.
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation
The Calorimeter Equation The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. To do so, the heat is exchanged with a calibrated object. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: When heat is absorbed by the solution, q for. Calorimetry is used to measure amounts of heat transferred to or from a substance. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Calculate the amount of heat released. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Use the equation for heat transfer \(q = mc\delta t\) to express the. Apply the first law of thermodynamics to calorimetry. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. Compare heat flow from hot to cold objects in an ideal. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation The Calorimeter Equation We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: When heat is absorbed by the solution, q for. The heat lost by the warm object is equal to the heat gained by the cooler. Calorimetry is used to measure amounts of heat transferred to or. The Calorimeter Equation.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry The Calorimeter Equation We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: Use the equation for heat transfer \(q = mc\delta t\) to express the. The heat lost by the warm object is equal to the heat gained by the cooler. Calorimetry is the measurement of the transfer. The Calorimeter Equation.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe The Calorimeter Equation The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Use the equation for heat transfer \(q = mc\delta t\) to express the. The heat lost by the warm object is equal to the heat gained by the cooler. To do so, the heat is exchanged with a calibrated object. Apply the first law of. The Calorimeter Equation.
From www.youtube.com
How to find calorimeter constant YouTube The Calorimeter Equation Apply the first law of thermodynamics to calorimetry. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\). The Calorimeter Equation.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube The Calorimeter Equation To do so, the heat is exchanged with a calibrated object. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. The equation for heat, q =. The Calorimeter Equation.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: The equation for heat, q = m x #c_s# x. The Calorimeter Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 The Calorimeter Equation To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Use the equation for heat transfer \(q = mc\delta t\) to express the. Apply the first law of thermodynamics to calorimetry. The equation for heat, q = m x #c_s# x #deltat# is used for. The Calorimeter Equation.
From www.youtube.com
Calorimetry Part 2 calculations YouTube The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: Then use equation \ (ref {5.5.9}\) to determine the heat. The Calorimeter Equation.
From www.youtube.com
Calorimetry calculation YouTube The Calorimeter Equation The heat lost by the warm object is equal to the heat gained by the cooler. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. When heat is absorbed by the solution, q for. Apply the first law of thermodynamics to calorimetry. Calculate the amount of heat. The Calorimeter Equation.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 The Calorimeter Equation The heat lost by the warm object is equal to the heat gained by the cooler. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: Calculate the amount of heat released. The equation for heat, q = m x #c_s# x #deltat# is used for. The Calorimeter Equation.
From www.youtube.com
Principle of Calorimetry YouTube The Calorimeter Equation Compare heat flow from hot to cold objects in an ideal. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Calculate the amount of heat released. Use the equation for heat transfer \(q = mc\delta t\) to express the. The heat lost by the warm object is equal to the heat gained by the. The Calorimeter Equation.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 The Calorimeter Equation To do so, the heat is exchanged with a calibrated object. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Calorimetry is used to measure amounts of heat transferred to. The Calorimeter Equation.
From chemistrytalk.org
Calorimetry ChemTalk The Calorimeter Equation Use the equation for heat transfer \(q = mc\delta t\) to express the. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry.. The Calorimeter Equation.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube The Calorimeter Equation When heat is absorbed by the solution, q for. Compare heat flow from hot to cold objects in an ideal. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). We can calculate the amount of heat absorbed by the solution or the amount of heat. The Calorimeter Equation.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). The heat lost by the warm object is equal to. The Calorimeter Equation.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube The Calorimeter Equation Use the equation for heat transfer \(q = mc\delta t\) to express the. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: Compare heat flow from hot to cold objects in an ideal. In this article, you will learn about calorimetry and calorimeters, mainly used. The Calorimeter Equation.
From www.numerade.com
SOLVEDThe defining equation for calorimetry is The Calorimeter Equation Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calculate the amount of heat released. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. To do so, the heat is. The Calorimeter Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download The Calorimeter Equation Use the equation for heat transfer \(q = mc\delta t\) to express the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object.. The Calorimeter Equation.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube The Calorimeter Equation The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. The heat lost by the warm object is equal to the heat gained by the cooler. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \. The Calorimeter Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download The Calorimeter Equation We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The heat lost by the pan is equal to the. The Calorimeter Equation.
From studymarxianism.z21.web.core.windows.net
How To Calculate Calorimeter The Calorimeter Equation When heat is absorbed by the solution, q for. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Use the equation for heat transfer \(q = mc\delta t\) to express the. The heat lost by the warm object is equal to the heat gained by the cooler. The. The Calorimeter Equation.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube The Calorimeter Equation Calculate the amount of heat released. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is the measurement of the transfer of. The Calorimeter Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 The Calorimeter Equation We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: Calculate the amount of heat released. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Apply the first law of thermodynamics. The Calorimeter Equation.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog The Calorimeter Equation The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Apply the first law of thermodynamics to. The Calorimeter Equation.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube The Calorimeter Equation We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: To do so, the heat is exchanged with a calibrated object. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_ {comb}\) and \ (δt\). Calorimetry. The Calorimeter Equation.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki The Calorimeter Equation Calculate the amount of heat released. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Apply the first law of thermodynamics to calorimetry. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of. The Calorimeter Equation.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog The Calorimeter Equation Use the equation for heat transfer \(q = mc\delta t\) to express the. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical. The Calorimeter Equation.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation: When heat is absorbed by the solution, q for. Compare. The Calorimeter Equation.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson The Calorimeter Equation Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Use the equation for heat transfer \(q = mc\delta t\) to express the. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. Compare heat flow from hot to cold objects in an. The Calorimeter Equation.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube The Calorimeter Equation Calculate the amount of heat released. To do so, the heat is exchanged with a calibrated object. Use the equation for heat transfer \(q = mc\delta t\) to express the. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. The equation for heat, q = m x #c_s#. The Calorimeter Equation.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a. The Calorimeter Equation.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry The Calorimeter Equation Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. The equation for heat, q = m x #c_s# x #deltat# is used for calorimetry. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calorimetry is. The Calorimeter Equation.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Then use equation \ (ref {5.5.9}\) to determine the heat capacity of the calorimeter (\ (c_ {bomb}\)) from \ (q_. The Calorimeter Equation.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors The Calorimeter Equation In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Use the equation for heat transfer \(q = mc\delta t\) to express the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. The Calorimeter Equation.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube The Calorimeter Equation Compare heat flow from hot to cold objects in an ideal. Use the equation for heat transfer \(q = mc\delta t\) to express the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. In this article,. The Calorimeter Equation.