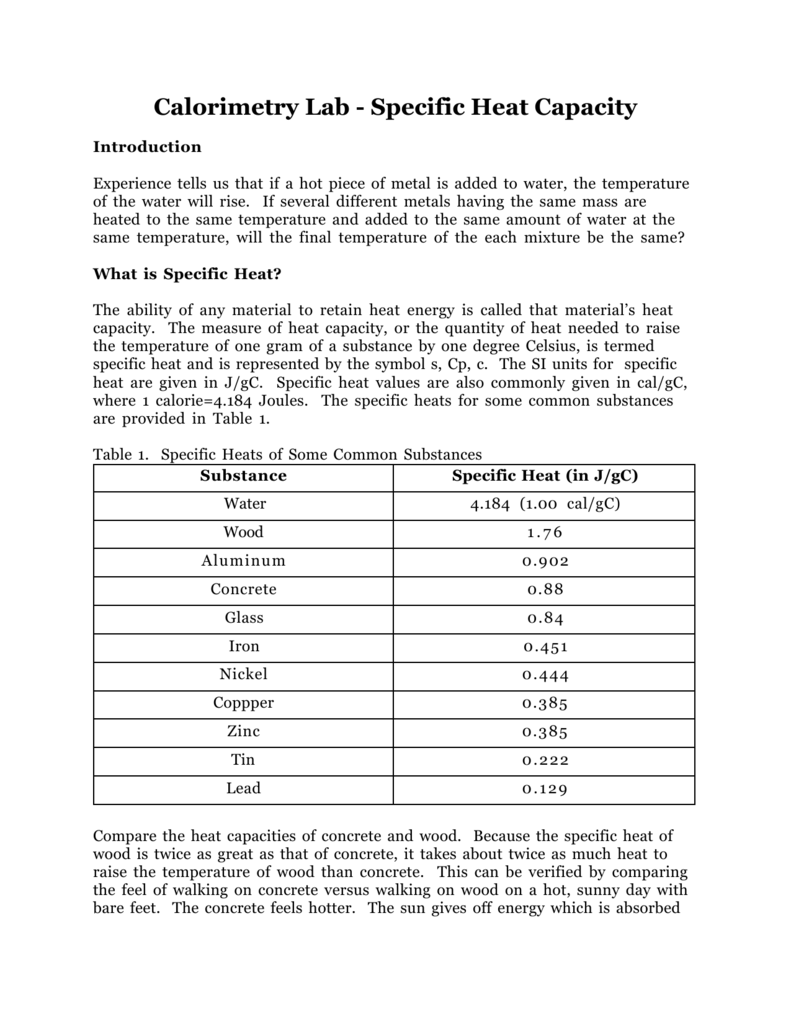
Calorimetry And Heat Capacity . q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties using typical calorimetry data. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Q = mcδt, where q is the. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat transfer and temperature change contains all three factors: — explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry.
from studylib.net
The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat transfer and temperature change contains all three factors: in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. Q = mcδt, where q is the. — explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data.
Calorimetry Lab Specific Heat Capacity
Calorimetry And Heat Capacity — the quantitative relationship between heat transfer and temperature change contains all three factors: Explain the technique of calorimetry. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = mcδt, where q is the. — explain the technique of calorimetry. — the quantitative relationship between heat transfer and temperature change contains all three factors:
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and related properties using typical. Q = mcδt, where q is the. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. — the quantitative relationship between heat transfer. Calorimetry And Heat Capacity.
From www.scribd.com
Calorimetry and Heat Capacity PDF Calorimetry And Heat Capacity Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. — the quantitative relationship between heat transfer and temperature change contains all three factors: Calculate and interpret heat and related properties using typical. — explain the technique of calorimetry. Explain the technique of. Calorimetry And Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry And Heat Capacity The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry.. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical calorimetry data. — the quantitative relationship between heat transfer and temperature change contains all three factors: in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. — explain the technique of calorimetry. The specific. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Section 7.2—Calorimetry & Heat Capacity PowerPoint Presentation Calorimetry And Heat Capacity The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical. — the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the. — explain the technique of calorimetry. . Calorimetry And Heat Capacity.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the.. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calculate and interpret heat and related properties using typical calorimetry data. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. Calculate and interpret heat and related properties using typical calorimetry data. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. in this article, you will learn about calorimetry and calorimeters, mainly used to. Calorimetry And Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry And Heat Capacity — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific. Calorimetry And Heat Capacity.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry And Heat Capacity The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt. Calorimetry And Heat Capacity.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry And Heat Capacity Q = mcδt, where q is the. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical calorimetry data. — the quantitative relationship between heat transfer and temperature change contains all three factors: Calculate and interpret heat and related. Calorimetry And Heat Capacity.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimetry And Heat Capacity — the quantitative relationship between heat transfer and temperature change contains all three factors: in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Explain the technique of calorimetry. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity Explain the technique of calorimetry. — explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g. Calorimetry And Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical calorimetry data. Q = mcδt, where q is the. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat transfer and temperature change contains all three factors: — explain the technique of. Calorimetry And Heat Capacity.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry And Heat Capacity — explain the technique of calorimetry. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Q = mcδt, where q is the. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or.. Calorimetry And Heat Capacity.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Calorimetry And Heat Capacity Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.. Calorimetry And Heat Capacity.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — explain the technique of calorimetry. — the quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat capacity (c) of a substance is. Calorimetry And Heat Capacity.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical. Q = mcδt, where q is the. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. — the quantitative relationship between heat. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry And Heat Capacity Explain the technique of calorimetry. — explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Calorimetry And Heat Capacity Q = mcδt, where q is the. Explain the technique of calorimetry. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Calculate and interpret heat and related properties using typical. — explain the technique of calorimetry. — the quantitative relationship between heat. Calorimetry And Heat Capacity.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. — the quantitative relationship between. Calorimetry And Heat Capacity.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — the quantitative relationship between heat transfer and temperature change contains all three factors: Calculate and interpret heat and related properties using typical calorimetry data. in this article, you. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical. Explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature of. Calorimetry And Heat Capacity.
From www.youtube.com
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples Calorimetry And Heat Capacity in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. — explain the technique of calorimetry. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat. Calorimetry And Heat Capacity.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry And Heat Capacity in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Explain the technique of calorimetry. — the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the. q = mcδt, where q is the. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID6765646 Calorimetry And Heat Capacity — the quantitative relationship between heat transfer and temperature change contains all three factors: — explain the technique of calorimetry. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. The specific heat capacity (c) of a substance is the heating required to. Calorimetry And Heat Capacity.
From dokumen.tips
(PPTX) Calorimetry Calorimetry is used to measure heat capacity and Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical calorimetry. Calorimetry And Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry And Heat Capacity Q = mcδt, where q is the. — explain the technique of calorimetry. — the quantitative relationship between heat transfer and temperature change contains all three factors: q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID3779058 Calorimetry And Heat Capacity q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — explain the technique of calorimetry. in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity in this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Q = mcδt, where q is the. — explain the technique of calorimetry. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the. Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID6765646 Calorimetry And Heat Capacity The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. Calculate and interpret heat and related properties using typical calorimetry data. — the quantitative relationship between heat transfer and temperature change contains all three factors: Explain the technique of calorimetry. Q = mcδt, where q is the. . Calorimetry And Heat Capacity.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimetry And Heat Capacity Calculate and interpret heat and related properties using typical. The specific heat capacity (c) of a substance is the heating required to raise the temperature of 1 g of a. — the quantitative relationship between heat transfer and temperature change contains all three factors: Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of. Calorimetry And Heat Capacity.