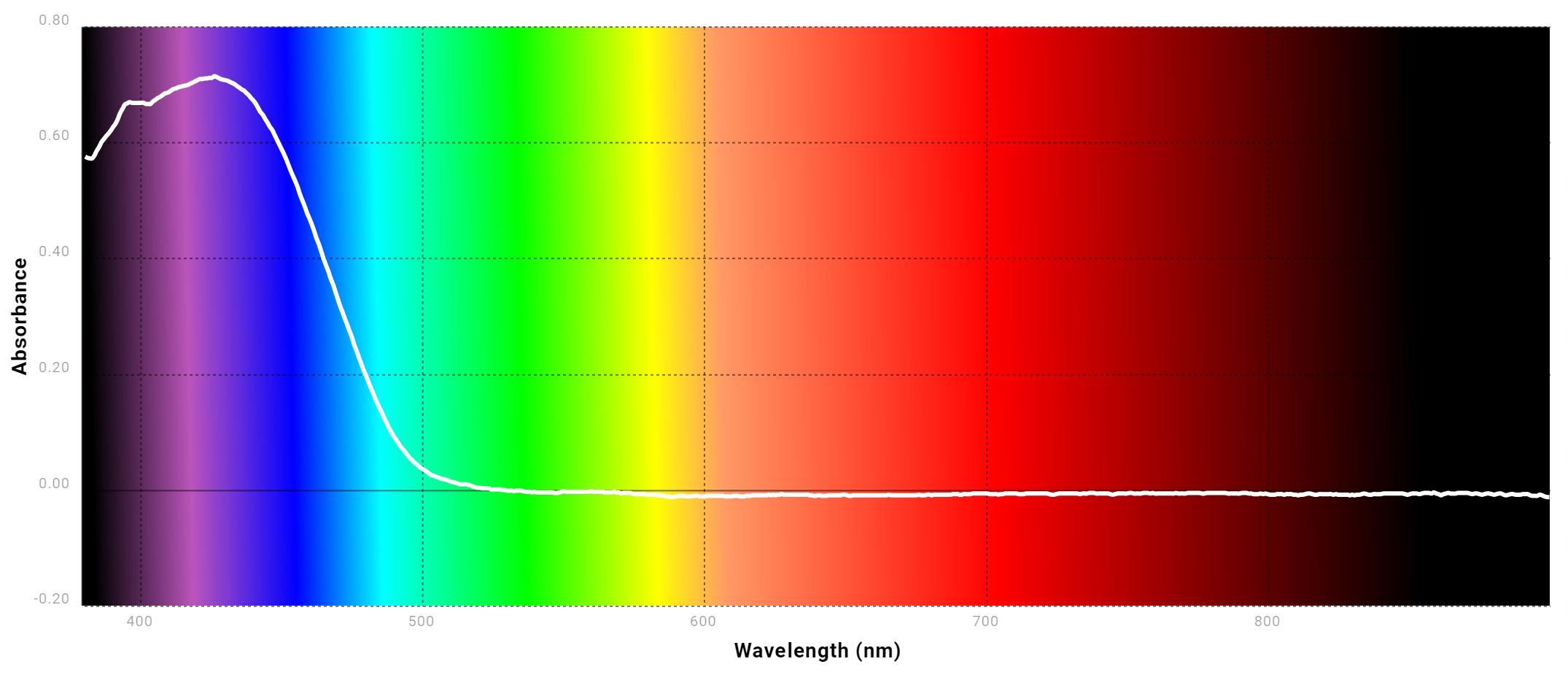
Spectrophotometry And Beer's Law Lab Report Chegg . The intensity of the color directly changes in response to the concentration. Use the given absorbance value of. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The intensity of the oolor imversely changes in response to the concentrabon. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. Introduction to spectrophotometer and beer’s law procedure: This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Here’s how to approach this question. 100% (6 ratings) share share. Beer’s law fabrizio chigne valerga lab partner: The goal of this experiment is to learn about beer's law by preparing.
from www.chegg.com
Introduction to spectrophotometer and beer’s law procedure: Use the given absorbance value of. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Beer’s law fabrizio chigne valerga lab partner: Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The goal of this experiment is to learn about beer's law by preparing. 100% (6 ratings) share share. This relationship is called the. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information.
SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE
Spectrophotometry And Beer's Law Lab Report Chegg Use the given absorbance value of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. 100% (6 ratings) share share. Introduction to spectrophotometer and beer’s law procedure: Beer’s law fabrizio chigne valerga lab partner: Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The goal of this experiment is to learn about beer's law by preparing. Here’s how to approach this question. Use the given absorbance value of. The intensity of the color directly changes in response to the concentration. The intensity of the oolor imversely changes in response to the concentrabon. This relationship is called the.
From www.chegg.com
Solved SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: Here’s how to approach this question. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The goal of this experiment is to learn about beer's law by preparing. 100% (6 ratings) share share. Identify the formatting aspects of the pictured. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Spectrophotometry And Beer's Law Lab Report Chegg 100% (6 ratings) share share. The intensity of the color directly changes in response to the concentration. This relationship is called the. Use the given absorbance value of. The intensity of the oolor imversely changes in response to the concentrabon. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Introduction to. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Dilutions, Spectroscopy, and Beer's Law OSTON Spectrophotometry And Beer's Law Lab Report Chegg 100% (6 ratings) share share. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The intensity of the color directly changes in response to the concentration. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The goal of this experiment is to. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Prelab LO7 Beer's Law (10pts) Name Please review the Spectrophotometry And Beer's Law Lab Report Chegg The goal of this experiment is to learn about beer's law by preparing. The intensity of the color directly changes in response to the concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Introduction to spectrophotometer and beer’s law procedure: Identify the formatting aspects of the. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
EXPERIMENT 5 SPECTROPHOTOMETRY AND BEER'S LAW The Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. 100% (6 ratings) share share. The intensity of the oolor imversely changes in response to the concentrabon. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Here’s how to approach this question. Introduction to spectrophotometer and beer’s law procedure:. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved SPECTROPHOTOMETRY. APPLY BEER'S LAW TO DETERMINE Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. 100% (6 ratings) share share. The intensity of the oolor imversely changes in response to the concentrabon. The goal of this experiment is to learn about beer's law by preparing. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium.. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law Spectrophotometry And Beer's Law Lab Report Chegg The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The intensity. Spectrophotometry And Beer's Law Lab Report Chegg.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Spectrophotometry And Beer's Law Lab Report Chegg Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. This relationship is called the. The intensity of the oolor imversely changes in response to the concentrabon. The goal of this experiment is to learn about beer's law by preparing. Identify the formatting aspects of the pictured beer's law plot graph that. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Wavelength 631.1 Absorbance 0.650 600 700 800 Spectrophotometry And Beer's Law Lab Report Chegg This relationship is called the. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Beer’s law fabrizio chigne valerga lab partner: The intensity of the color directly changes in response to the concentration. Here’s how to approach this question. The goal of this experiment is to learn about beer's law by. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Spectrophotometry and Beer's Law spectrophotometry Spectrophotometry And Beer's Law Lab Report Chegg The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Introduction to spectrophotometer and beer’s law procedure: 100% (6 ratings) share share. Beer’s law fabrizio chigne valerga lab partner: The goal of this. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Spectrophotometry and Beer's Law estra inte metery Spectrophotometry And Beer's Law Lab Report Chegg The goal of this experiment is to learn about beer's law by preparing. The intensity of the color directly changes in response to the concentration. This relationship is called the. Here’s how to approach this question. Introduction to spectrophotometer and beer’s law procedure: The pre lab report on page 22 of my lab notebook shows the general procedure used during. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Determination of the Equilibrium Spectrophotometry And Beer's Law Lab Report Chegg Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Here’s how to approach this question. Use the given absorbance value of. The intensity of the oolor imversely changes in response to the concentrabon. The intensity of the color directly changes in response to the concentration. Experiment 5 spectrophotometry and beer's law. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Beer’s Law and Spectrophotometry Lab Experiment Title Beer’s Law and Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Use the given absorbance value of. Introduction to spectrophotometer and beer’s law procedure: The intensity of the oolor imversely changes in response to the concentrabon. This relationship is called the.. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Example lab report spectrophotometry lab BS2205AUCO Essex Studocu Spectrophotometry And Beer's Law Lab Report Chegg Here’s how to approach this question. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The intensity of the color directly changes in response to the concentration. Beer’s law fabrizio chigne valerga lab partner: The pre lab report on page 22 of my lab notebook shows the general procedure used during. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: 100% (6 ratings) share share. Introduction to spectrophotometer and beer’s law procedure: Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The intensity of the oolor imversely changes in response to the concentrabon. The amount of light that a species absorbs in a spectroscopic. Spectrophotometry And Beer's Law Lab Report Chegg.
From future4200.com
Getting started in analytical chemistry Spectrophotometry and Analysis Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. The intensity of the oolor imversely changes in response to the concentrabon. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. 100% (6 ratings) share share. Identify the formatting aspects of the pictured beer's law plot graph that. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Spectrophotometry and Beer's Law spremenities Lab Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. 100% (6 ratings) share share. This relationship is called the. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Use the given absorbance value of. The amount of light that a species absorbs in a spectroscopic transition can be. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Spectrophotometry And Beer's Law Lab Report Chegg 100% (6 ratings) share share. Use the given absorbance value of. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. This relationship is called the. Here’s how to approach this question. Beer’s law fabrizio chigne valerga lab partner: Identify the formatting aspects of the pictured beer's law plot graph that contain. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. Here’s how to approach this question. 100% (6 ratings) share share. The intensity of the color. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
CHE144 Lab Manual Experiments 1 EXPERIMENT ONE Spectrophotometry and Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the color directly changes in response to the concentration. 100% (6 ratings) share share. Here’s how to approach this question. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Beer’s law fabrizio chigne valerga lab partner: The intensity of the oolor imversely changes in response to the concentrabon.. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.youtube.com
Beer Lambert Law, Molar Extinction Coefficient, Spectrophotometry YouTube Spectrophotometry And Beer's Law Lab Report Chegg Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. 100% (6 ratings) share share. Here’s how to approach this question. Introduction to spectrophotometer and beer’s law procedure: The goal of this experiment is to learn about beer's law by preparing. The amount of light that a species absorbs in a spectroscopic. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Spectrophotometry And Beer's Law Lab Report Chegg This relationship is called the. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Here’s how to approach this question. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. The amount of light that a species absorbs in a spectroscopic transition. Spectrophotometry And Beer's Law Lab Report Chegg.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The goal of this experiment is to learn about beer's law by preparing. 100% (6 ratings) share share. This relationship is called the. Here’s how to approach this question. The pre lab report on page. Spectrophotometry And Beer's Law Lab Report Chegg.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Spectrophotometry And Beer's Law Lab Report Chegg 100% (6 ratings) share share. This relationship is called the. The goal of this experiment is to learn about beer's law by preparing. The intensity of the oolor imversely changes in response to the concentrabon. The intensity of the color directly changes in response to the concentration. Beer’s law fabrizio chigne valerga lab partner: Use the given absorbance value of.. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved spectrophotometry and Beer's Law Concentration of Cr' Spectrophotometry And Beer's Law Lab Report Chegg Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. The goal of this experiment is to learn about beer's law by preparing. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. Beer’s law fabrizio chigne valerga lab partner: Introduction to spectrophotometer and. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved spectrophotometry and Beer's Law Concentration of Cr' Spectrophotometry And Beer's Law Lab Report Chegg Here’s how to approach this question. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Introduction to spectrophotometer and beer’s law procedure: Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of chromium. This relationship is called the. Identify the. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Spectrophotometry and Beer's Law spremenities Lab Spectrophotometry And Beer's Law Lab Report Chegg 100% (6 ratings) share share. The goal of this experiment is to learn about beer's law by preparing. Here’s how to approach this question. Introduction to spectrophotometer and beer’s law procedure: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Experiment 5 spectrophotometry and beer's law the. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Lab Name BeerLambert Law And Spectroscopy Data S... Spectrophotometry And Beer's Law Lab Report Chegg The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. 100% (6 ratings) share share. Here’s how to approach this question. Beer’s law fabrizio chigne valerga lab partner: The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. The. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Lab 1 Spectrophotometry Report Pang Mei Chen 26076055 BioChem 3506 Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The goal of this experiment is to learn about beer's law by preparing. The intensity of the color directly changes in response to the concentration. 100% (6 ratings) share share. Here’s how to approach this. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Simulation Data and Report Submission Beer's Law Spectrophotometry And Beer's Law Lab Report Chegg The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The intensity of the oolor imversely changes in response to the concentrabon. 100% (6 ratings) share share. Beer’s law fabrizio chigne valerga lab partner: Use the given absorbance value of. The intensity of the color directly changes in. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Spectrophotometry And Beer's Law Lab Report Chegg Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The intensity of the oolor imversely changes in response to the concentrabon. Beer’s law fabrizio chigne valerga lab partner: 100% (6 ratings) share share. Introduction to spectrophotometer and beer’s law procedure: The goal of this experiment is to learn about beer's law. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
SPECTROPHOTOMETRY • APPLY BEER'S LAW TO DETERMINE Spectrophotometry And Beer's Law Lab Report Chegg The goal of this experiment is to learn about beer's law by preparing. Beer’s law fabrizio chigne valerga lab partner: The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. Here’s how to approach this question. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.chegg.com
Solved Determining the Concentration of a Solution Beer's Spectrophotometry And Beer's Law Lab Report Chegg Introduction to spectrophotometer and beer’s law procedure: The goal of this experiment is to learn about beer's law by preparing. Use the given absorbance value of. Here’s how to approach this question. Identify the formatting aspects of the pictured beer's law plot graph that contain errors, such as missing information. The intensity of the oolor imversely changes in response to. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Spectrophotometry And Beer's Law Lab Report Chegg Beer’s law fabrizio chigne valerga lab partner: 100% (6 ratings) share share. The goal of this experiment is to learn about beer's law by preparing. Use the given absorbance value of. The pre lab report on page 22 of my lab notebook shows the general procedure used during this experiment. This relationship is called the. The intensity of the oolor. Spectrophotometry And Beer's Law Lab Report Chegg.
From www.studocu.com
Spectrophotometry Lab MT Virtual Lab Report Spectrophotometry Learn Spectrophotometry And Beer's Law Lab Report Chegg The intensity of the oolor imversely changes in response to the concentrabon. The goal of this experiment is to learn about beer's law by preparing. 100% (6 ratings) share share. Beer’s law fabrizio chigne valerga lab partner: Here’s how to approach this question. Experiment 5 spectrophotometry and beer's law the purpose of this experiment is to determine the concentration of. Spectrophotometry And Beer's Law Lab Report Chegg.