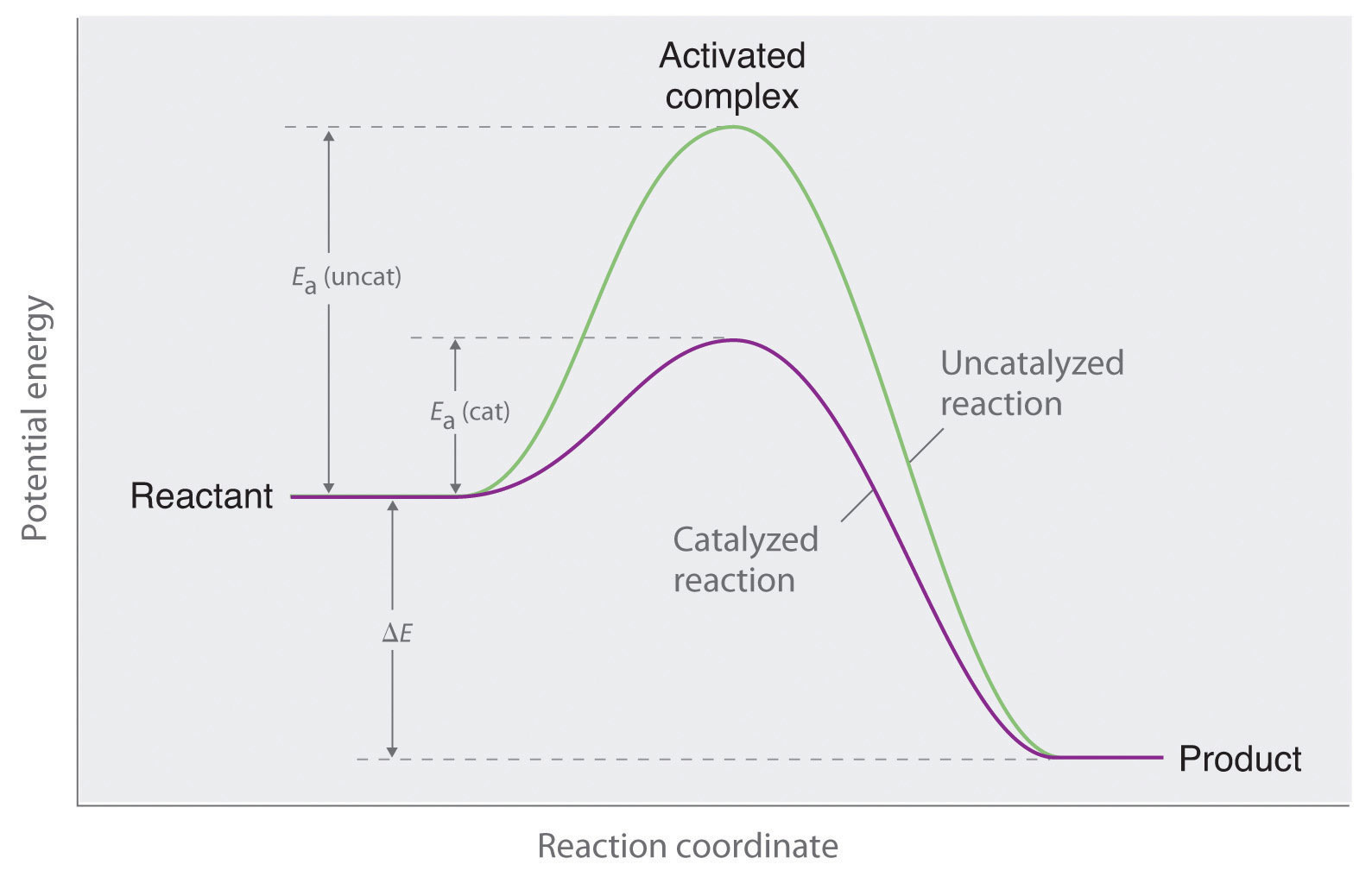
A Catalyst Cannot Affect . Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. A catalysed reaction produces the same amount of product. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts are usually specific to a particular reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. The only effect of the. However, not all reactions have suitable catalysts. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the.
from chem.libretexts.org
Only a very small mass of catalyst is needed to increase the rate of a reaction. The best catalyst for one reaction is unlikely to have any effect at all on a different. However, not all reactions have suitable catalysts. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts are usually specific to a particular reaction. A catalysed reaction produces the same amount of product.
Chapter 14.8 Catalysis Chemistry LibreTexts
A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. The best catalyst for one reaction is unlikely to have any effect at all on a different. A catalysed reaction produces the same amount of product. The only effect of the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. However, not all reactions have suitable catalysts. Catalysts are usually specific to a particular reaction. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. However, not all reactions have suitable catalysts. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Only a very small mass of catalyst is needed to increase. A Catalyst Cannot Affect.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction A Catalyst Cannot Affect The only effect of the. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalysed reaction produces the same amount of product. Catalysts are usually specific to a particular reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. This is because a. A Catalyst Cannot Affect.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. Catalysts have no effect on the equilibrium constant. A Catalyst Cannot Affect.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii A Catalyst Cannot Affect A catalysed reaction produces the same amount of product. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The only effect of the. The best catalyst for one reaction is unlikely to have any effect at all on a. A Catalyst Cannot Affect.
From kunduz.com
[ANSWERED] A catalyst cannot affect A Product B Rate of reaction C Kunduz A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The best catalyst for one reaction. A Catalyst Cannot Affect.
From www.numerade.com
SOLVED The presence of a catalyst decreases the activation energy of a A Catalyst Cannot Affect Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts are usually specific to a particular reaction. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. However, not all. A Catalyst Cannot Affect.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of A Catalyst Cannot Affect Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalysed reaction produces the same amount of product. Catalysts are usually specific to a particular reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts act by reducing the amount of activation energy needed for a successful. A Catalyst Cannot Affect.
From www.numerade.com
SOLVEDUse graphs to illustrate how the presence of a catalyst can A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. The best catalyst for one reaction is unlikely to have any effect at all on a different. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. However, not all. A Catalyst Cannot Affect.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 A Catalyst Cannot Affect A catalysed reaction produces the same amount of product. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts are usually specific to a particular reaction. The best catalyst for one reaction is unlikely to have any effect at all on a different. Only a very small mass of. A Catalyst Cannot Affect.
From hxebmwake.blob.core.windows.net
A Catalyst Lowers The Activation Energy But Does Not Affect The A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. However, not all reactions have suitable. A Catalyst Cannot Affect.
From chembam.com
Catalysts A Catalyst Cannot Affect The only effect of the. The best catalyst for one reaction is unlikely to have any effect at all on a different. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules.. A Catalyst Cannot Affect.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. However, not all reactions have suitable catalysts. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts are usually specific to. A Catalyst Cannot Affect.
From ppt-online.org
Rates of reaction презентация онлайн A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. Catalysts are usually specific to a particular reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. The best catalyst for one reaction is. A Catalyst Cannot Affect.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 A Catalyst Cannot Affect Catalysts are usually specific to a particular reaction. The only effect of the. The best catalyst for one reaction is unlikely to have any effect at all on a different. Only a very small mass of catalyst is needed to increase the rate of a reaction. Because adding a catalyst doesn't affect the relative rates of the two reactions, it. A Catalyst Cannot Affect.
From slideplayer.com
CATALYST “Energy cannot be created or destroyed but can be converted A Catalyst Cannot Affect Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Only a very small mass of catalyst is. A Catalyst Cannot Affect.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. The best catalyst for one reaction is unlikely to have any effect at all on a different. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. A catalysed reaction produces the same amount. A Catalyst Cannot Affect.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation, free download ID676158 A Catalyst Cannot Affect Only a very small mass of catalyst is needed to increase the rate of a reaction. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts act by reducing the amount of activation energy needed. A Catalyst Cannot Affect.
From askfilo.com
EXERCISE 14. A catalyst cannot change Filo A Catalyst Cannot Affect Catalysts are usually specific to a particular reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalysed reaction produces the same amount of product. This is because a catalyst speeds up the forward. A Catalyst Cannot Affect.
From www.youtube.com
7.2.4 State and explain the effect of a catalyst on an equilibrium A Catalyst Cannot Affect Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts act by reducing the amount of activation energy. A Catalyst Cannot Affect.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 A Catalyst Cannot Affect The only effect of the. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. A catalysed reaction produces the same amount of product. Only a. A Catalyst Cannot Affect.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in A Catalyst Cannot Affect Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The best catalyst for one reaction is unlikely to have any effect at all on a different. The only effect of the.. A Catalyst Cannot Affect.
From slidetodoc.com
ENZYME BIOLOGICAL CATALYST Enzyme As Catalyst All enzymes A Catalyst Cannot Affect A catalysed reaction produces the same amount of product. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. The best catalyst for one reaction is unlikely to have any effect at all on a different. The only effect of the. Catalysts have no effect on the equilibrium constant and thus on. A Catalyst Cannot Affect.
From ammoniaknowhow.com
Catalyst deactivation Common causes AmmoniaKnowHow A Catalyst Cannot Affect Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts are usually specific to a particular reaction. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the. A Catalyst Cannot Affect.
From www.numerade.com
⏩SOLVEDDoes a catalyst affect the value of the equilibrium… Numerade A Catalyst Cannot Affect The only effect of the. A catalysed reaction produces the same amount of product. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts are usually specific to a particular reaction. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. However, not. A Catalyst Cannot Affect.
From www.numerade.com
SOLVED Two catalysts are being analyzed to determine how they affect A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. A catalysed reaction produces the same amount of product. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Only a very small mass of catalyst is needed to increase the rate. A Catalyst Cannot Affect.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect A Catalyst Cannot Affect A catalysed reaction produces the same amount of product. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. However, not. A Catalyst Cannot Affect.
From byjus.com
Why do catalyst not affect equilibrium? A Catalyst Cannot Affect The only effect of the. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. However, not all reactions have suitable catalysts. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts have no effect on the equilibrium constant and thus on the equilibrium. A Catalyst Cannot Affect.
From chem.libretexts.org
Chapter 14.8 Catalysis Chemistry LibreTexts A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The only effect of the. Catalysts are usually specific to a particular reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Only a very small mass of catalyst is needed. A Catalyst Cannot Affect.
From slideplayer.com
Physical Science Chapter 7, Section 3 Types of Chemical Reactions A Catalyst Cannot Affect The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts are usually specific to a particular reaction. However, not all reactions have suitable catalysts. Because adding a catalyst doesn't affect the relative rates of the two reactions, it. A Catalyst Cannot Affect.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review A Catalyst Cannot Affect However, not all reactions have suitable catalysts. The only effect of the. Catalysts are usually specific to a particular reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts act by reducing the amount of. A Catalyst Cannot Affect.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis A Catalyst Cannot Affect This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. The best catalyst for one reaction is unlikely to have any effect at all on a different. Catalysts are usually specific to a particular reaction. However, not all reactions have suitable catalysts. Because adding a catalyst. A Catalyst Cannot Affect.
From www.brainyquote.com
Top 10 Catalyst Quotes BrainyQuote A Catalyst Cannot Affect Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Catalysts are usually specific to a particular reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition.. A Catalyst Cannot Affect.
From hxeaspeqb.blob.core.windows.net
Why Do Catalysts Not Affect The Position Of Equilibrium at Erica Murphy A Catalyst Cannot Affect The only effect of the. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. However, not all reactions have suitable catalysts. The best catalyst for one reaction is unlikely to have any effect at all. A Catalyst Cannot Affect.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint A Catalyst Cannot Affect Catalysts are usually specific to a particular reaction. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. However, not all reactions have suitable catalysts. This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the. Catalysts. A Catalyst Cannot Affect.
From slideplayer.com
Equilibrium Chapter ppt download A Catalyst Cannot Affect The best catalyst for one reaction is unlikely to have any effect at all on a different. Because adding a catalyst doesn't affect the relative rates of the two reactions, it can't affect the position of equilibrium. Catalysts are usually specific to a particular reaction. Only a very small mass of catalyst is needed to increase the rate of a. A Catalyst Cannot Affect.