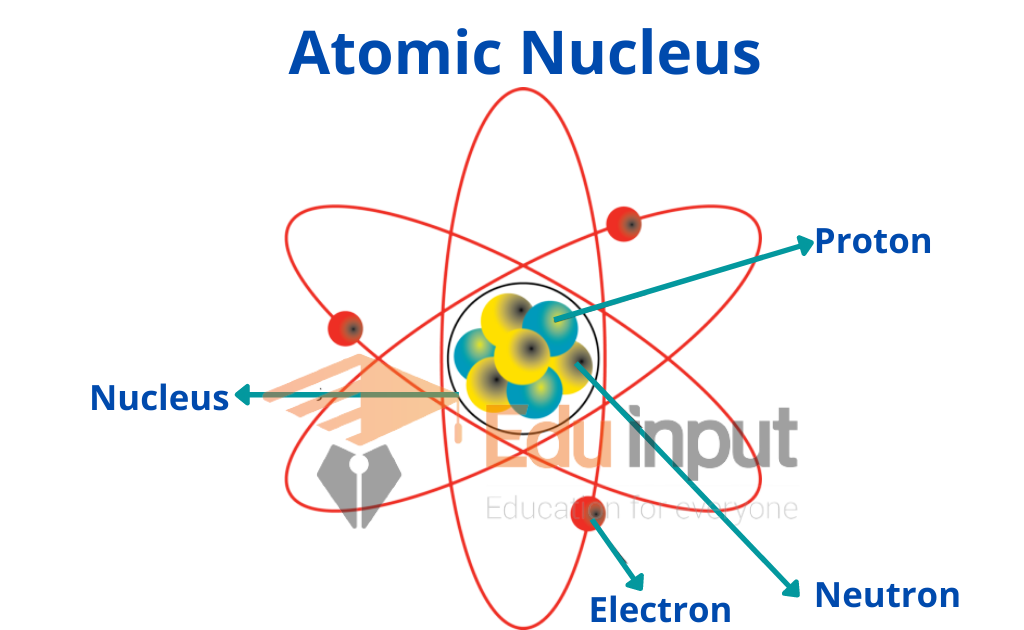
Letter/Number Of Nucleus . Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. Here are two ways of writing nuclear symbols, along with examples. What is nuclear symbol notation? Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The mass of the atom is a unit called the. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. The list is ordered by increasing atomic number, which is the number of. Oxygen and carbon are both elements. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Atomic number and mass number.
from eduinput.com
What is nuclear symbol notation? The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Here are two ways of writing nuclear symbols, along with examples. The list is ordered by increasing atomic number, which is the number of. Atomic number and mass number. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The mass of the atom is a unit called the. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Oxygen and carbon are both elements. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element.
Atomic Nucleus Nucleons, Charge Number, and Mass Number
Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Here are two ways of writing nuclear symbols, along with examples. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. What is nuclear symbol notation? Oxygen and carbon are both elements. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the. The list is ordered by increasing atomic number, which is the number of. Atomic number and mass number. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols.
From hubpages.com
Cell components and their functions HubPages Letter/Number Of Nucleus 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. The mass of the atom is a unit called the. Both are made up of atoms. Letter/Number Of Nucleus.
From www.researchgate.net
Total nuclei numbers and allocations of nuclei (mean 6 SD) to ICM and Letter/Number Of Nucleus Here are two ways of writing nuclear symbols, along with examples. Oxygen and carbon are both elements. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of. Letter/Number Of Nucleus.
From brainly.in
describe structure and function of nucleus with the help of diagram Letter/Number Of Nucleus Both are made up of atoms which in turn are made up of protons, neutrons and electrons. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Oxygen and carbon are both elements. What is nuclear symbol notation? Atomic number and mass number. Basically, the nuclear symbol is a type of shorthand. Letter/Number Of Nucleus.
From sciencenotes.org
What Are Nucleons? Definition and Examples Letter/Number Of Nucleus Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Atomic number and mass number. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The chart of the nuclides shows the known nuclei in terms of. Letter/Number Of Nucleus.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definitions.. and more Letter/Number Of Nucleus Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. The list is ordered by increasing atomic number, which is the number of. Atomic number and mass number. What is nuclear symbol notation? The mass number (a a) of an atom is the total. Letter/Number Of Nucleus.
From www.scienceabc.com
Nucleus Function What Is A Nucleus? What Does The Nucleus Do? Letter/Number Of Nucleus The mass of the atom is a unit called the. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The chart of the nuclides shows the. Letter/Number Of Nucleus.
From sciencing.com
Nucleus Definition, Structure & Function (with Diagram) Sciencing Letter/Number Of Nucleus Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Here are two ways of writing nuclear symbols, along with examples. Atomic number and. Letter/Number Of Nucleus.
From studylib.net
Electron Configuration Practice Worksheet Letter/Number Of Nucleus The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. The list is ordered by increasing atomic number, which is the number of. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Here. Letter/Number Of Nucleus.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Scientists distinguish between different. Letter/Number Of Nucleus.
From guidedehartsicklewort.z21.web.core.windows.net
Draw A Labeled Diagram Of A Nucleus Letter/Number Of Nucleus The mass of the atom is a unit called the. Oxygen and carbon are both elements. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols.. Letter/Number Of Nucleus.
From www.nuclear-power.com
Atomic and Nuclear Structure Definition & Characteristics nuclear Letter/Number Of Nucleus What is nuclear symbol notation? The list is ordered by increasing atomic number, which is the number of. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Atomic number and mass number. The mass number (a a) of an atom is the total number of protons and neutrons in. Letter/Number Of Nucleus.
From eduinput.com
Atomic Nucleus Nucleons, Charge Number, and Mass Number Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the. Atomic number and mass number. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Both are made up of atoms which in. Letter/Number Of Nucleus.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Letter/Number Of Nucleus Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. The list is ordered by increasing atomic number, which is the number of. The chart. Letter/Number Of Nucleus.
From www.researchgate.net
1 MAGIC NUMBERS OF ATOMS, NUCLEI, AND THE MHO FROM Download Table Letter/Number Of Nucleus Oxygen and carbon are both elements. Atomic number and mass number. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. 119 rows here is a list of elements of the periodic table, their. Letter/Number Of Nucleus.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Letter/Number Of Nucleus The mass of the atom is a unit called the. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Here are two ways of writing nuclear symbols, along with examples.. Letter/Number Of Nucleus.
From studylib.net
26.1 Properties of nucleus Letter/Number Of Nucleus The list is ordered by increasing atomic number, which is the number of. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Atomic number and mass number. The mass of the atom is a unit called the. Scientists distinguish between different elements by the atomic number (z z), which. Letter/Number Of Nucleus.
From www.slideserve.com
PPT The Atomic Nucleus PowerPoint Presentation, free download ID Letter/Number Of Nucleus The mass of the atom is a unit called the. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The list is ordered by increasing. Letter/Number Of Nucleus.
From courses.lumenlearning.com
Nuclear Equations Chemistry Letter/Number Of Nucleus Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Oxygen and carbon are both elements. What is nuclear symbol notation? Basically, the nuclear symbol is a type of shorthand notation that identifies the. Letter/Number Of Nucleus.
From www.slideserve.com
PPT Nuclear / Subatomic Physics PowerPoint Presentation, free Letter/Number Of Nucleus 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Atomic number and mass number. What is nuclear symbol notation? Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Both are made up of atoms. Letter/Number Of Nucleus.
From sciencenotes.org
Learn the Parts of an Atom Letter/Number Of Nucleus What is nuclear symbol notation? The mass of the atom is a unit called the. Here are two ways of writing nuclear symbols, along with examples. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Atomic number and mass number. The chart of the nuclides shows the known nuclei in terms. Letter/Number Of Nucleus.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] Letter/Number Of Nucleus What is nuclear symbol notation? Oxygen and carbon are both elements. The list is ordered by increasing atomic number, which is the number of. Atomic number and mass number. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Both are made up of. Letter/Number Of Nucleus.
From stock.adobe.com
Atomic elements showing the nucleus and shells, numbers of electrons Letter/Number Of Nucleus Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. 119 rows here is a list of elements. Letter/Number Of Nucleus.
From www.slideserve.com
PPT Chapter 12 Atoms & the Periodic Table PowerPoint Presentation Letter/Number Of Nucleus 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Atomic number and mass number. The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Here are two ways of writing nuclear symbols, along with examples. Basically, the nuclear symbol is a type. Letter/Number Of Nucleus.
From exploringnature.org
Cell Organelle Matching Letter/Number Of Nucleus 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. Atomic number and mass number. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons. Letter/Number Of Nucleus.
From animalia-life.club
Atomic Nucleus Letter/Number Of Nucleus Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The mass number (a a) of an atom is the total number of protons and neutrons in. Letter/Number Of Nucleus.
From studylib.net
X A Z Nuclear Notation Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. What is nuclear symbol notation? 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The. Letter/Number Of Nucleus.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram Letter/Number Of Nucleus Here are two ways of writing nuclear symbols, along with examples. The list is ordered by increasing atomic number, which is the number of. Atomic number and mass number. What is nuclear symbol notation? Both are made up of atoms which in turn are made up of protons, neutrons and electrons. 119 rows here is a list of elements of. Letter/Number Of Nucleus.
From www.youtube.com
Counting Nuclei or Cell numbers from H and E Immunohistochemistry Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Both are made. Letter/Number Of Nucleus.
From www.britannica.com
Atom Proton, Neutron, Nucleus Britannica Letter/Number Of Nucleus Oxygen and carbon are both elements. The mass of the atom is a unit called the. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The list is ordered by increasing atomic number, which is the number of. Here are two ways of. Letter/Number Of Nucleus.
From microbenotes.com
Nucleus Definition, Structure, Parts, Functions, Diagram Letter/Number Of Nucleus The mass number (a a) of an atom is the total number of protons and neutrons in its nucleus. Scientists distinguish between different elements by the atomic number (z z), which represents the number of protons in the nucleus of one atom of that element. The list is ordered by increasing atomic number, which is the number of. Here are. Letter/Number Of Nucleus.
From www.vedantu.com
Nucleus Cell Nucleus Nucleus Structure and Functions Letter/Number Of Nucleus 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Here are two ways of writing nuclear symbols, along with examples. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. Scientists distinguish between different elements. Letter/Number Of Nucleus.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Letter/Number Of Nucleus Oxygen and carbon are both elements. What is nuclear symbol notation? Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. Here are two ways of. Letter/Number Of Nucleus.
From courses.lumenlearning.com
The Nucleus and DNA Replication Anatomy and Physiology I Letter/Number Of Nucleus Oxygen and carbon are both elements. What is nuclear symbol notation? The list is ordered by increasing atomic number, which is the number of. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. 119 rows here is a list of elements of the. Letter/Number Of Nucleus.
From www.slideserve.com
PPT Which letter represents the protons? PowerPoint Presentation Letter/Number Of Nucleus Oxygen and carbon are both elements. Basically, the nuclear symbol is a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element. The list is ordered by increasing atomic number, which is the number of. The chart of the nuclides shows the known nuclei in terms of their atomic number,. Letter/Number Of Nucleus.
From education.myheritage.com
Genealogy Glossary Common DNA Terms Explained MyHeritage Knowledge Base Letter/Number Of Nucleus Oxygen and carbon are both elements. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The chart of the nuclides shows the known nuclei in terms of their atomic number, z, and neutron number, n. What is nuclear symbol notation? Scientists distinguish between different elements by the atomic number (z z), which. Letter/Number Of Nucleus.