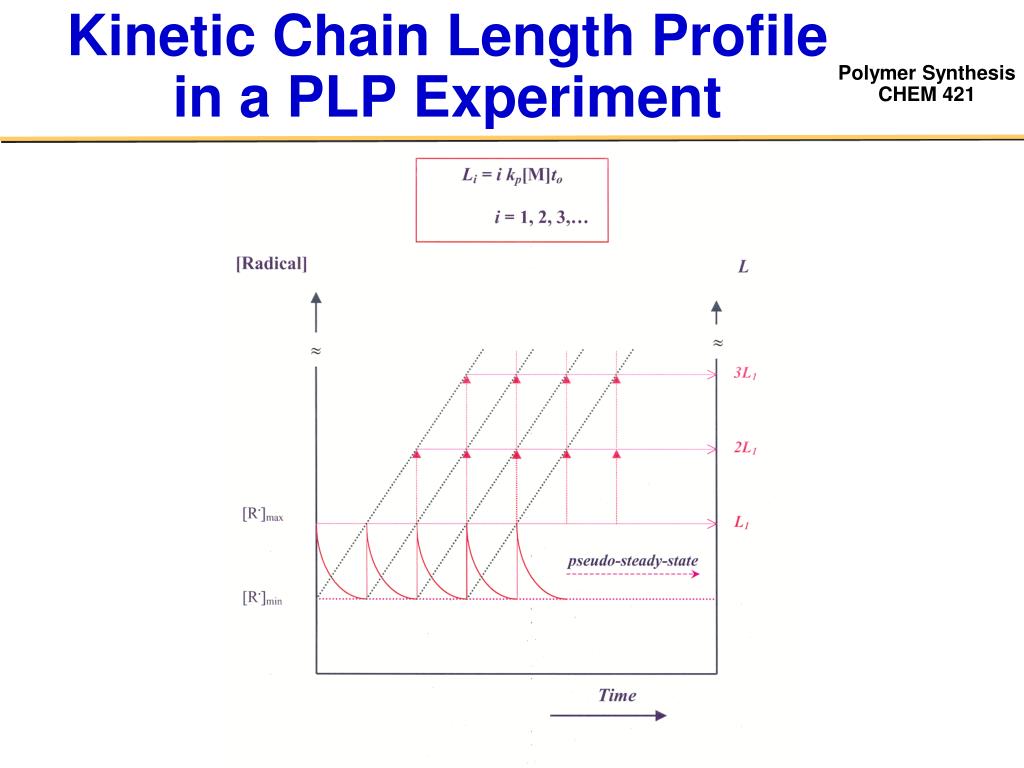
Kinetic Chain Length . Ν = rate of chain growth = rate of chain growth. That should tell us the number of. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar.
from www.slideserve.com
Ν = rate of chain growth = rate of chain growth. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. This quantity is called kinetic chain length, represented by vee bar. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Ν = # of monomers added per effective free radical. That should tell us the number of. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;.
PPT Free Radical Polymerizations PowerPoint Presentation, free
Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. Ν = rate of chain growth = rate of chain growth. That should tell us the number of. Ν = # of monomers added per effective free radical.
From marinagoldsmith.com.sg
Find Your Fit Necklace Chain Length Guide Marina Goldsmith Kinetic Chain Length Ν = # of monomers added per effective free radical. That should tell us the number of. This quantity is called kinetic chain length, represented by vee bar. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. Ν = rate of chain growth = rate of chain. Kinetic Chain Length.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID254280 Kinetic Chain Length Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The average number of monomers reacting. Kinetic Chain Length.
From slainte.usli.com
The Chain Explained Fundamentals of Proper Movement USLI Kinetic Chain Length Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Ν = # of monomers added. Kinetic Chain Length.
From www.pinterest.ph
PPT Chain Exercises Open vs . Closed Chain PowerPoint Kinetic Chain Length This quantity is called kinetic chain length, represented by vee bar. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Ν = rate of chain growth = rate of chain growth. Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center. Kinetic Chain Length.
From www.slideserve.com
PPT Chap 9. Chaingrowth Polymerization PowerPoint Presentation, free Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. That should tell us the number of. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Ν = rate of chain growth = rate of chain growth. Kinetic. Kinetic Chain Length.
From www.slideserve.com
PPT Ionic polymerization PowerPoint Presentation, free download ID Kinetic Chain Length Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. In principle, it's just. Kinetic Chain Length.
From www.slideserve.com
PPT Chap 9. Chaingrowth Polymerization PowerPoint Presentation, free Kinetic Chain Length Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! The. Kinetic Chain Length.
From www.mashelite.com
Must Add Accessory Movements Mash Elite Performance Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The kinetic chain length,. Kinetic Chain Length.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID382916 Kinetic Chain Length The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. That should tell us the number of. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Ν = # of monomers added per effective free radical. The average number of monomers. Kinetic Chain Length.
From www.slideserve.com
PPT INTRODUCTION TO FREE RADICAL POLYMERIZATION PowerPoint Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length, v̄, in a polymerization is defined as the average number of. Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = rate of chain. Kinetic Chain Length.
From www.slideserve.com
PPT KINESIOLOGY PowerPoint Presentation, free download ID6544945 Kinetic Chain Length That should tell us the number of. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Ν = rate of chain growth = rate of chain growth. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. This quantity is called. Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System Kinetic Chain Length Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! This quantity is called kinetic chain length, represented by vee bar. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length, v̄, in a polymerization. Kinetic Chain Length.
From www.youtube.com
Introduction to Polymers Lecture 6.6 Free radical polymerization Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Kinetic chain length. Kinetic Chain Length.
From bodyinbalancept.com
The Concept of the Chain Body in Balance PT Kinetic Chain Length Ν = # of monomers added per effective free radical. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Ν = rate of chain growth = rate of chain growth. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! That should tell us the number. Kinetic Chain Length.
From www.slideserve.com
PPT Ionic, RingOpening, & Controlled Polymerization PowerPoint Kinetic Chain Length Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! Ν = # of monomers added per effective free radical. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length, v̄, in a polymerization is. Kinetic Chain Length.
From www.researchgate.net
(PDF) Loaded exercises stretch the anterior cruciate Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. That should tell us the number of. Ν = rate of chain growth = rate of chain growth. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which. Kinetic Chain Length.
From posturegeek.com
Open Chain vs Closed Chain What's the Difference? Kinetic Chain Length Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. That should tell. Kinetic Chain Length.
From www.slideserve.com
PPT Free Radical Polymerizations PowerPoint Presentation, free Kinetic Chain Length That should tell us the number of. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar. Ν = rate of chain growth = rate of chain. Kinetic Chain Length.
From www.chegg.com
Solved How will the degree of polymerization in cationic Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. This quantity is called kinetic chain length, represented by vee bar. Ν = # of. Kinetic Chain Length.
From www.slideshare.net
Polymer Course Kinetic Chain Length The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. That should tell us the number of. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! The average number of monomers reacting with a given active center from its initiations. Kinetic Chain Length.
From strengthandsoul.co.uk
What is Chain Release (KCR), can it help you? And why do we Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. That should tell us the number of. The kinetic chain length, v̄,. Kinetic Chain Length.
From www.youtube.com
Chain Chain Length中文翻译 YouTube Kinetic Chain Length This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Ν = rate of chain growth = rate of chain growth. Ν. Kinetic Chain Length.
From www.slideserve.com
PPT Engineering aspect of emulsion polymerization PowerPoint Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. The kinetic chain length, v̄, in a polymerization is defined as the average number of. Kinetic Chain Length.
From www.topvelocity.net
Mastering The High Velocity Pitchers Chain TopVelocity Kinetic Chain Length Ν = # of monomers added per effective free radical. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is. Kinetic Chain Length.
From www.researchgate.net
Variation of the parameters k 0 and λ with the DNA chain length Kinetic Chain Length The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. Ν = rate of chain growth = rate of chain growth. This quantity is called kinetic chain length, represented by vee bar. Ν = # of monomers added per effective free radical. The average number of monomers reacting. Kinetic Chain Length.
From www.youtube.com
(ENGLISH) MECHANISM CATIONIC ADDITION DEGREE OF POLYMERIZATION Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = # of monomers added per effective free radical. This quantity is called kinetic chain length, represented by vee bar. The average number of monomers reacting with a given active center from its initiations to its termination is. Kinetic Chain Length.
From www.slideserve.com
PPT Ionic, RingOpening, & Controlled Polymerization PowerPoint Kinetic Chain Length The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. This quantity is called kinetic chain length, represented by vee bar. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Kinetic chain length refers to the average number of repeating units. Kinetic Chain Length.
From www.youtube.com
Polymer chemistry। Flory theory। chain length। physIcal Kinetic Chain Length Ν = # of monomers added per effective free radical. That should tell us the number of. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Ν = rate of chain growth = rate of chain growth. Mechanism of radical chain polymerization fundamental steps (initiation,. Kinetic Chain Length.
From velocityspusa.com
The Chain in Overhead Sports A Linked System Kinetic Chain Length Ν = rate of chain growth = rate of chain growth. The kinetic chain length, v̄, in a polymerization is defined as the average number of growth reactions which follow an initiation process;. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Kinetic chain length. Kinetic Chain Length.
From www.youtube.com
of Polymerization and Average Chain Length YouTube Kinetic Chain Length The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! This quantity is called kinetic chain length, represented by vee bar. Kinetic chain length refers to the average number. Kinetic Chain Length.
From www.massagemag.com
The 5 Primary Chains Poster Set (5 charts) Products Directory Kinetic Chain Length That should tell us the number of. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! This quantity is called kinetic chain length, represented by vee bar. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Kinetic chain length refers to the average number of. Kinetic Chain Length.
From www.kineticprobaseball.com
Unit 3 The Chain Kinetic Chain Length Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. Ν = rate of chain growth = rate of chain growth. The average number of monomers reacting with a given active center from its initiations to its termination is represented by n, that is kinetic. This quantity is called. Kinetic Chain Length.
From www.slideshare.net
Chains • Open Chain Kinetic Chain Length Ν = # of monomers added per effective free radical. That should tell us the number of. This quantity is called kinetic chain length, represented by vee bar. Mechanism of radical chain polymerization fundamental steps (initiation, propagation, termination) kinetic chain length and molecular weight ! In principle, it's just the ratio of the chain propagation rate to the chain initiation. Kinetic Chain Length.
From www.youtube.com
Thermal Initiation (Contd.), Molecular Weight and Chain Length Kinetic Chain Length This quantity is called kinetic chain length, represented by vee bar. That should tell us the number of. Kinetic chain length refers to the average number of repeating units or monomers that are incorporated into a growing polymer chain. In principle, it's just the ratio of the chain propagation rate to the chain initiation rate. Mechanism of radical chain polymerization. Kinetic Chain Length.