What Is Medical Device Management . Medical devices in particular are crucial for safe and effective: Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Management and safe use of medical devices health technologies are essential for a functioning health system. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso).
from www.greenlight.guru
Management and safe use of medical devices health technologies are essential for a functioning health system. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices in particular are crucial for safe and effective: It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso).
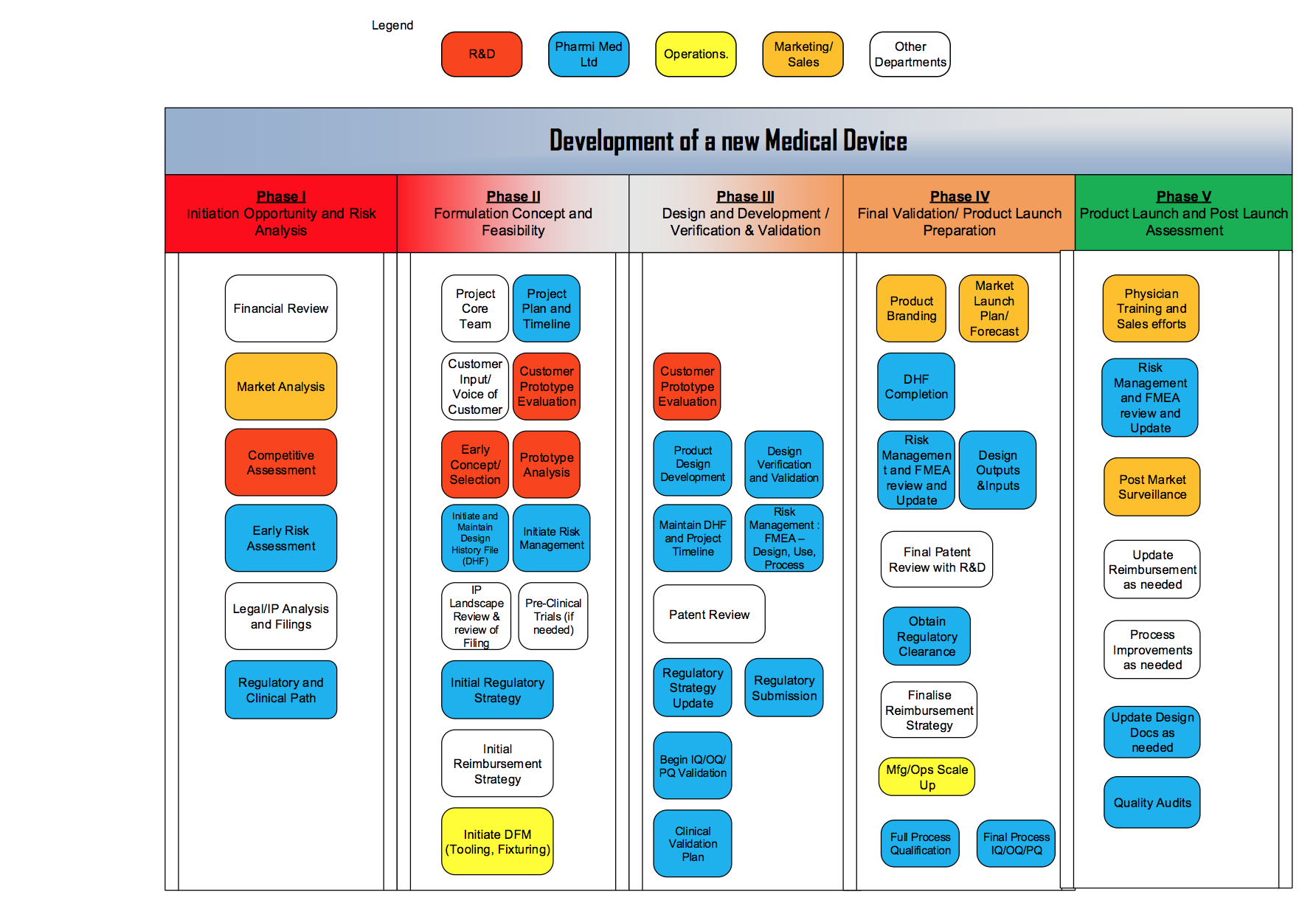
Understanding the 5 Phases of Medical Device Development
What Is Medical Device Management Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Management and safe use of medical devices health technologies are essential for a functioning health system. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices in particular are crucial for safe and effective: Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. What Is Medical Device Management Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. It is. What Is Medical Device Management.
From www.iso-certs.co.uk
MEDICAL DEVICES MANAGEMENT SYSTEM ISO CERT INTERNATIONAL LTD What Is Medical Device Management Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in.. What Is Medical Device Management.
From www.dotcompliance.com
Quality Management System (QMS) for Medical Device Dot Compliance What Is Medical Device Management Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Management and safe use of medical devices health technologies are essential for a functioning health system. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. It is intended primarily. What Is Medical Device Management.
From www.universityofgalway.ie
Medical Device Management University of Galway What Is Medical Device Management Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Medical devices in. What Is Medical Device Management.
From kantify.com
Kantify Improving Human Health through Artificial Intelligence What Is Medical Device Management Medical devices in particular are crucial for safe and effective: Management and safe use of medical devices health technologies are essential for a functioning health system. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance,. What Is Medical Device Management.
From financesonline.com
Best Medical Practice Management Software in 2024 What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Management. What Is Medical Device Management.
From www.birlasoft.com
Acing Master Data Management in Medical Device Industry What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. A medical device manufacturer’s quality management system. What Is Medical Device Management.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co What Is Medical Device Management It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Medical devices in particular are crucial for safe and effective: Iso 13485 is the global standard for medical device quality. What Is Medical Device Management.
From www.researchgate.net
The LifeCycle of a Medical Device. Download Scientific Diagram What Is Medical Device Management Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Management and safe use of medical devices health technologies are essential for a functioning health system. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485:2016 specifies requirements. What Is Medical Device Management.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development What Is Medical Device Management Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Management and safe use of medical devices health technologies are essential for a functioning health system. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485:2016 specifies requirements. What Is Medical Device Management.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key What Is Medical Device Management Medical devices in particular are crucial for safe and effective: A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485:2016 specifies requirements for a quality management system where. What Is Medical Device Management.
From www.jamasoftware.com
What is Medical Device Risk Management? Blog What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485 is the. What Is Medical Device Management.
From www.slideshare.net
6 Ways to Improve Your Medical Device Management What Is Medical Device Management A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical. What Is Medical Device Management.
From www.complianceonline.com
ISO 149712019 Medical devices Application of Risk Management to What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical devices in particular are crucial for safe and effective: Management and safe use of medical devices health technologies are essential for a functioning health system. It is intended primarily for people in hospital and community based organisations that are responsible. What Is Medical Device Management.
From sunstonepilot.com
Introduction to Medical Device Development Sunstone Pilot, Inc. What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Quality management for. What Is Medical Device Management.
From www.researchgate.net
Medical device lifecycle management (see online version for colours What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. Medical devices in particular are crucial for safe and effective: Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate. What Is Medical Device Management.
From medicaldevicehq.com
Medical device project management Medical Device HQ What Is Medical Device Management It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices in particular are crucial for safe and effective: Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Iso 13485:2016 specifies requirements for a quality management system where. What Is Medical Device Management.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development What Is Medical Device Management Medical devices in particular are crucial for safe and effective: Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. It is intended primarily for people in hospital and community based. What Is Medical Device Management.
From www.vrogue.co
Choosing The Right Medical Device Risk Management Too vrogue.co What Is Medical Device Management Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Management. What Is Medical Device Management.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito What Is Medical Device Management It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. A medical. What Is Medical Device Management.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog What Is Medical Device Management A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Medical. What Is Medical Device Management.
From pharmait.dk
Medical Device Quality Management System processes Pharma IT What Is Medical Device Management It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are.. What Is Medical Device Management.
From worldcomplianceseminars.com
Medical Device Quality Management System Course WCS What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Management and safe use of medical devices health technologies are essential for a functioning health system. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485:2016 specifies requirements for. What Is Medical Device Management.
From www.youtube.com
Automating Medical Device Management YouTube What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Quality management for medical devices refers. What Is Medical Device Management.
From www.autymate.com
Healthcare Device Management What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Management and safe use of medical devices health technologies are essential for a functioning health system. Quality management for medical devices. What Is Medical Device Management.
From www.qualitymeddev.com
IEC 62304 Medical Device Software Overview of the Main Requirements What Is Medical Device Management Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical devices in. What Is Medical Device Management.
From floridamedicaldeviceconsultant.blogspot.com
Get the Medical Device Consultant With an Incredible Service Since Day one What Is Medical Device Management A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Medical. What Is Medical Device Management.
From www.slideteam.net
Medical Device Development And Commercialization Process PPT PowerPoint What Is Medical Device Management It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many. What Is Medical Device Management.
From www.syscreations.com
Medical Device Integration Solutions With EMR, EHR, LIS, HIS What Is Medical Device Management Medical devices in particular are crucial for safe and effective: It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Management and safe use of medical devices health technologies are essential for a functioning health system. Quality management for medical devices refers to the systems and processes put in place. What Is Medical Device Management.
From www.vrogue.co
The 12 Phases Of Medical Device Development Meridian vrogue.co What Is Medical Device Management Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. It is intended primarily for people in hospital and community based organisations that are responsible for the management of medical. Medical devices in particular are crucial for safe and effective: Iso 13485:2016 specifies requirements for a quality management system where. What Is Medical Device Management.
From timly.com
Medical Device Management in Healthcare Sector Made Easy What Is Medical Device Management Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Quality management for medical devices refers to the systems and processes put in place to ensure that medical devices are. Management and safe use of medical devices health technologies are essential for a functioning health system. A medical device manufacturer’s quality. What Is Medical Device Management.
From www.ebme.co.uk
Medical devices getting management policies right What Is Medical Device Management A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many. What Is Medical Device Management.
From www.cognidox.com
4 ways to build a medical device quality management system What Is Medical Device Management A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Iso 13485 is the global standard for medical device quality management systems established by the international standards organization (iso). Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. It is intended. What Is Medical Device Management.
From correctiveactionsoftware.com
Medical Device Management Qualitech Solutions What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. A medical device manufacturer’s quality management system is the foundation for maintaining regulatory compliance, driving improvement, effectiveness and achieving stakeholder. Quality management for medical devices refers. What Is Medical Device Management.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing What Is Medical Device Management Management and safe use of medical devices health technologies are essential for a functioning health system. Iso 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Medical devices in particular are crucial. What Is Medical Device Management.