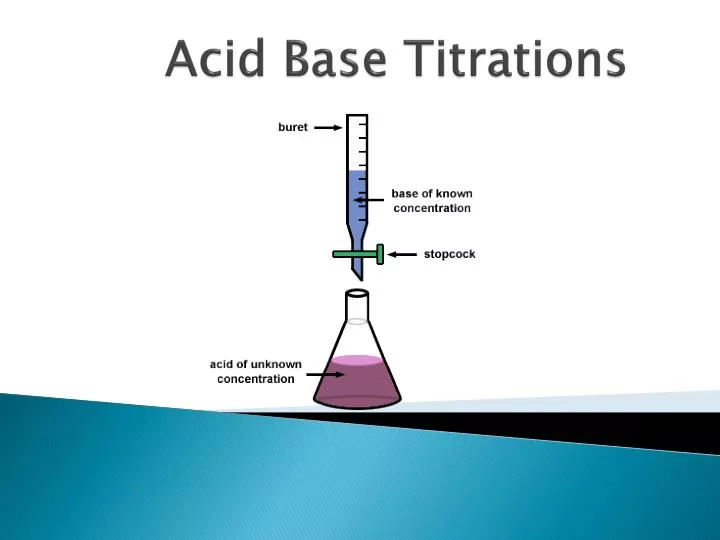
Why Is Titration Accurate . Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. While classical titration was performed by manually. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. The basic process involves adding a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from www.slideserve.com
The basic process involves adding a. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. While classical titration was performed by manually. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why.
PPT Acid Base Titrations PowerPoint Presentation, free download ID
Why Is Titration Accurate While classical titration was performed by manually. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. While classical titration was performed by manually. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. For accuracy, a titration reaction should be fast, complete, unambiguous and observable.
From www.science-revision.co.uk
Titrations Why Is Titration Accurate When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. The basic process involves adding a. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a volumetric technique in which a. Why Is Titration Accurate.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Why Is Titration Accurate A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the. Why Is Titration Accurate.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. The basic process involves adding a. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common method of quantifying the presence of an analyte in a sample is through titration, remaining. Why Is Titration Accurate.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. While classical titration was performed by manually. The basic process involves adding a. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common. Why Is Titration Accurate.
From pages.hannainst.com
Titration Best Practices Improving Accuracy of Your Titration Results Why Is Titration Accurate While classical titration was performed by manually. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but. Why Is Titration Accurate.
From theedge.com.hk
Chemistry How To Titration The Edge Why Is Titration Accurate When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A common method. Why Is Titration Accurate.
From themasterchemistry.com
Why Is Titration Important In Chemistry? Themasterchemistry Why Is Titration Accurate A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common method. Why Is Titration Accurate.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Why Is Titration Accurate While classical titration was performed by manually. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. For accuracy, a titration. Why Is Titration Accurate.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Why Is Titration Accurate Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the. Why Is Titration Accurate.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Why Is Titration Accurate Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. While classical titration was performed by manually. For accuracy, a titration reaction should be. Why Is Titration Accurate.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Why Is Titration Accurate The basic process involves adding a. While classical titration was performed by manually. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of. Why Is Titration Accurate.
From mmerevise.co.uk
Titrations and Uncertainties MME Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the. Why Is Titration Accurate.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common method. Why Is Titration Accurate.
From www.microlit.com
An Advanced Guide to Titration Microlit Why Is Titration Accurate A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. Method validation of a titration ensures that the selected titration method and parameters will provide a. Why Is Titration Accurate.
From www.analytica-world.com
Titration Handbook Theory and Practice of Titration A guide to Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A titration is a volumetric technique in which a. Why Is Titration Accurate.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Why Is Titration Accurate Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. A titration is a volumetric technique in which a solution of. Why Is Titration Accurate.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. Method validation of a titration ensures that the selected. Why Is Titration Accurate.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. While classical titration was performed by manually. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common method of quantifying the presence of an analyte in a sample is through titration,. Why Is Titration Accurate.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Why Is Titration Accurate The basic process involves adding a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. While classical. Why Is Titration Accurate.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. While classical titration was performed by manually. When. Why Is Titration Accurate.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry Why Is Titration Accurate While classical titration was performed by manually. The basic process involves adding a. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to. Why Is Titration Accurate.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an Why Is Titration Accurate Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. While classical titration was performed by manually. A. Why Is Titration Accurate.
From relationshipbetween.com
Difference Between Titration And Neutralization Relationship Between Why Is Titration Accurate The basic process involves adding a. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. While classical titration was performed by manually. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Why Is Titration Accurate.
From gamma.app
Understanding Titration Why Is Titration Accurate While classical titration was performed by manually. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Why Is Titration Accurate.
From bramblechemistry.weebly.com
4C6 Titration Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Why Is Titration Accurate.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. While classical titration was performed by manually. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A. Why Is Titration Accurate.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Why Is Titration Accurate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Method validation of a titration ensures that the. Why Is Titration Accurate.
From themasterchemistry.com
Why Is Titration Important In Chemistry? Themasterchemistry Why Is Titration Accurate A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the technique. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Why Is Titration Accurate.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset Why Is Titration Accurate Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries. Why Is Titration Accurate.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Why Is Titration Accurate For accuracy, a titration reaction should be fast, complete, unambiguous and observable. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The basic process involves adding a. Method validation of a titration ensures that the selected titration. Why Is Titration Accurate.
From www.scribd.com
Titration PDF Titration Chemistry Why Is Titration Accurate While classical titration was performed by manually. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. A common method of quantifying the presence of an analyte in a sample is through titration, remaining relevant for centuries thanks to the simplicity and accuracy of the. Why Is Titration Accurate.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Why Is Titration Accurate When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. For accuracy, a titration reaction should be fast, complete, unambiguous and observable. The basic process involves adding a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Why Is Titration Accurate.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Why Is Titration Accurate The basic process involves adding a. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see. Why Is Titration Accurate.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Why Is Titration Accurate When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a. Why Is Titration Accurate.
From byjus.com
Why is titration used? Why Is Titration Accurate When we do titrations (w/ phenolphthalein) in lab, i know it's fun to see how close we can get the solution to clear, but why. The basic process involves adding a. While classical titration was performed by manually. Method validation of a titration ensures that the selected titration method and parameters will provide a reliable and robust result. A common. Why Is Titration Accurate.