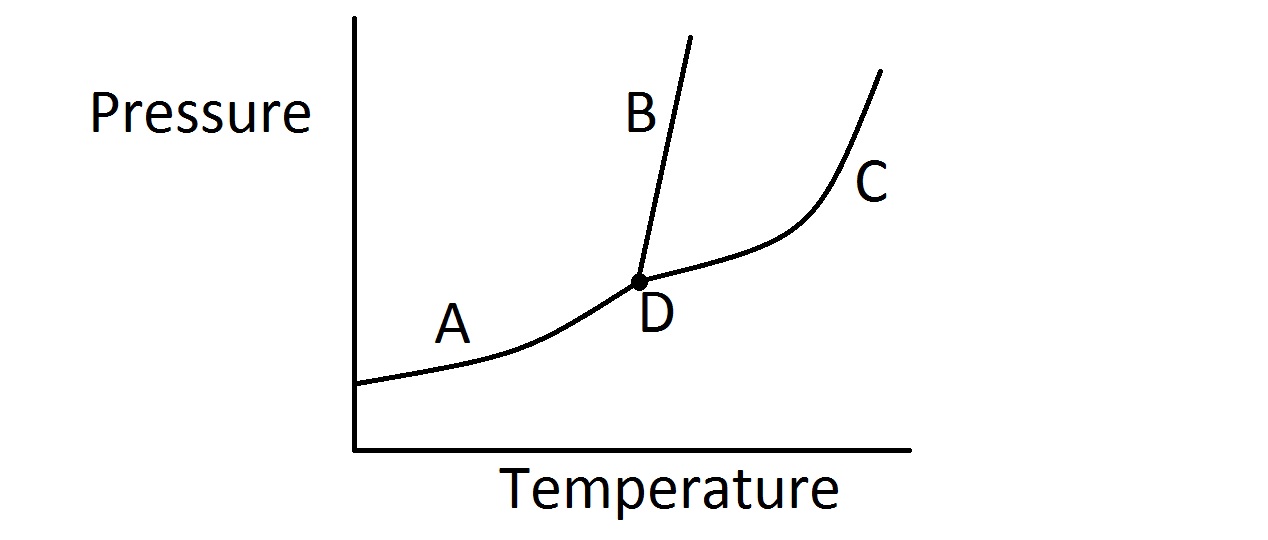
Phase Diagram Vertical Line . To be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. Several distinct lines separate these phases. This section discusses some common kinds of binary systems, and sec. This simple diagram tells you roughly how the system behaves. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. 13.3 will describe some interesting ternary systems. For most compounds, this line has a small positive slope, as shown in the. It’s called the phase line. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases.
from www.varsitytutors.com
For most compounds, this line has a small positive slope, as shown in the. 13.3 will describe some interesting ternary systems. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. It’s called the phase line. This section discusses some common kinds of binary systems, and sec. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. This simple diagram tells you roughly how the system behaves. To be able to identify the triple point, the critical point, and four regions:
Phase Diagrams College Chemistry
Phase Diagram Vertical Line 13.3 will describe some interesting ternary systems. This simple diagram tells you roughly how the system behaves. To be able to identify the triple point, the critical point, and four regions: It’s called the phase line. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Several distinct lines separate these phases. For most compounds, this line has a small positive slope, as shown in the. This section discusses some common kinds of binary systems, and sec. 13.3 will describe some interesting ternary systems. Solid, liquid, gas, and a supercritical fluid. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. The phase line captures exactly the information we.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. For most compounds, this line has a small positive slope, as shown in the. The phase line captures exactly the information we. Solid, liquid, gas, and a supercritical fluid. This simple diagram. Phase Diagram Vertical Line.
From scientifictutor.org
Chem Phase Diagrams Scientific Tutor Phase Diagram Vertical Line It’s called the phase line. This section discusses some common kinds of binary systems, and sec. 13.3 will describe some interesting ternary systems. Several distinct lines separate these phases. This simple diagram tells you roughly how the system behaves. For most compounds, this line has a small positive slope, as shown in the. The bd line is almost vertical because. Phase Diagram Vertical Line.
From www.youtube.com
Phase Line Diagrams Math Modelling Lecture 11 YouTube Phase Diagram Vertical Line This simple diagram tells you roughly how the system behaves. Solid, liquid, gas, and a supercritical fluid. 13.3 will describe some interesting ternary systems. For most compounds, this line has a small positive slope, as shown in the. Several distinct lines separate these phases. This section discusses some common kinds of binary systems, and sec. It’s called the phase line.. Phase Diagram Vertical Line.
From pressbooks.bccampus.ca
2.3 Phase diagrams Introduction to Engineering Thermodynamics Phase Diagram Vertical Line To be able to identify the triple point, the critical point, and four regions: The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Solid, liquid, gas, and a supercritical fluid. 13.3 will describe some interesting ternary systems. For most compounds, this line has a small positive slope, as. Phase Diagram Vertical Line.
From chem.libretexts.org
Phase Diagrams Chemistry LibreTexts Phase Diagram Vertical Line These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. This section discusses some common kinds of binary systems, and sec. This simple diagram tells you roughly how the system behaves. It’s called the phase line. The phase line captures exactly the information we. Several distinct lines separate these phases. The bd line. Phase Diagram Vertical Line.
From serc.carleton.edu
Phase Rule Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Several distinct lines separate these phases. It’s called the phase line. The phase line captures exactly the information we. To be able to identify the triple point, the. Phase Diagram Vertical Line.
From physics.stackexchange.com
thermodynamics Chemical equilibrium on phase diagram Physics Stack Phase Diagram Vertical Line For most compounds, this line has a small positive slope, as shown in the. This section discusses some common kinds of binary systems, and sec. This simple diagram tells you roughly how the system behaves. 13.3 will describe some interesting ternary systems. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. Several. Phase Diagram Vertical Line.
From diagramdataconley.z5.web.core.windows.net
How To Read Phase Diagram Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. This simple diagram tells you roughly how the system behaves. Several distinct lines. Phase Diagram Vertical Line.
From pressbooks.bccampus.ca
2.3 Phase diagrams Introduction to Engineering Thermodynamics Phase Diagram Vertical Line 13.3 will describe some interesting ternary systems. It’s called the phase line. To be able to identify the triple point, the critical point, and four regions: The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. The phase line captures exactly the information we. This section discusses some common. Phase Diagram Vertical Line.
From unistudium.unipg.it
Phase Diagrams Phase Diagram Vertical Line For most compounds, this line has a small positive slope, as shown in the. 13.3 will describe some interesting ternary systems. The phase line captures exactly the information we. Solid, liquid, gas, and a supercritical fluid. It’s called the phase line. This section discusses some common kinds of binary systems, and sec. To be able to identify the triple point,. Phase Diagram Vertical Line.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry Phase Diagram Vertical Line The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. For most compounds, this line has a small positive slope, as shown in the. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. To be able to identify the triple point,. Phase Diagram Vertical Line.
From academy.gwb.com
The Geochemist's Workbench® Online Academy Phase diagrams Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: The phase line captures exactly the information we. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in. Phase Diagram Vertical Line.
From www.youtube.com
Basic Points for Drawing Phase Diagram How to Draw Phase diagram Phase Diagram Vertical Line These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. For most compounds, this line has a small positive slope, as shown in the. This section discusses some common kinds of binary systems, and sec. The phase line captures exactly the information we. To be able to identify the triple point, the critical. Phase Diagram Vertical Line.
From chem.libretexts.org
13.2 Phase Diagrams Binary Systems Chemistry LibreTexts Phase Diagram Vertical Line The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. This section discusses some common kinds of binary systems, and sec. Solid, liquid, gas, and a supercritical fluid. This simple diagram tells you roughly how the system behaves. 13.3 will describe some interesting ternary systems. For most compounds, this. Phase Diagram Vertical Line.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. It’s called the phase line. To be able to identify the triple point, the critical point, and four regions: This simple diagram tells you roughly how the system behaves. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. For most compounds,. Phase Diagram Vertical Line.
From www.varsitytutors.com
Phase Diagrams College Chemistry Phase Diagram Vertical Line For most compounds, this line has a small positive slope, as shown in the. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. This simple diagram tells you roughly how the system behaves. To be able to identify the triple point, the critical point, and four regions: Solid,. Phase Diagram Vertical Line.
From www.researchgate.net
A phase diagram showing approximate scalings for the convective flux F Phase Diagram Vertical Line This simple diagram tells you roughly how the system behaves. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. It’s called the phase line. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. Several distinct lines separate these phases. For most compounds, this line has a. Phase Diagram Vertical Line.
From www.researchgate.net
Phase diagrams in the (g, λ) plane. We use N = 1 and N = 10 for the Phase Diagram Vertical Line Several distinct lines separate these phases. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. This section discusses some common kinds of binary systems, and sec. 13.3 will describe some interesting ternary systems.. Phase Diagram Vertical Line.
From www.chem.fsu.edu
Phase Diagrams Phase Diagram Vertical Line 13.3 will describe some interesting ternary systems. To be able to identify the triple point, the critical point, and four regions: This simple diagram tells you roughly how the system behaves. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. These lines are called lines of equilibrium or. Phase Diagram Vertical Line.
From wirepartpate.z13.web.core.windows.net
How To Read A Phase Change Diagram Phase Diagram Vertical Line This simple diagram tells you roughly how the system behaves. 13.3 will describe some interesting ternary systems. Solid, liquid, gas, and a supercritical fluid. It’s called the phase line. The phase line captures exactly the information we. To be able to identify the triple point, the critical point, and four regions: The bd line is almost vertical because the melting. Phase Diagram Vertical Line.
From glossary.periodni.com
Phase diagram Chemistry Dictionary & Glossary Phase Diagram Vertical Line These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. It’s called the phase line. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. This section discusses some common kinds of binary systems, and sec. For most compounds, this line has a small positive slope, as shown. Phase Diagram Vertical Line.
From courses.lumenlearning.com
Phase Diagrams Chemistry Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. This simple diagram tells you roughly how the system behaves. 13.3. Phase Diagram Vertical Line.
From www.jove.com
Phase Diagram JoVE Phase Diagram Vertical Line This simple diagram tells you roughly how the system behaves. It’s called the phase line. Solid, liquid, gas, and a supercritical fluid. This section discusses some common kinds of binary systems, and sec. 13.3 will describe some interesting ternary systems. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in. Phase Diagram Vertical Line.
From chem.libretexts.org
13.3 Phase Diagrams of Phases Systems Phase Diagram Vertical Line Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. For most compounds, this line has a small positive slope, as shown in the. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. 13.3 will describe some interesting ternary systems. To be able to identify the triple. Phase Diagram Vertical Line.
From www.researchgate.net
Ternary phase diagram of CuAlNi, vertical cross section at 3 mass Phase Diagram Vertical Line To be able to identify the triple point, the critical point, and four regions: For most compounds, this line has a small positive slope, as shown in the. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. Solid, liquid, gas, and a supercritical fluid. 13.3 will describe some interesting ternary systems. This. Phase Diagram Vertical Line.
From www.engineeringprep.com
Binary Phase Diagram Engineering Prep Phase Diagram Vertical Line To be able to identify the triple point, the critical point, and four regions: 13.3 will describe some interesting ternary systems. The phase line captures exactly the information we. Solid, liquid, gas, and a supercritical fluid. For most compounds, this line has a small positive slope, as shown in the. The bd line is almost vertical because the melting point. Phase Diagram Vertical Line.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Phase Diagram Vertical Line The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. To be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. 13.3. Phase Diagram Vertical Line.
From solvedlib.com
Consider the phase diagram below If the dashed line … SolvedLib Phase Diagram Vertical Line These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. The phase line captures exactly the information we. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Solid, liquid, gas, and a supercritical fluid. Several distinct lines separate these phases. To. Phase Diagram Vertical Line.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Phase Diagram Vertical Line For most compounds, this line has a small positive slope, as shown in the. 13.3 will describe some interesting ternary systems. Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: This section discusses some common kinds of binary systems, and sec. It’s called the phase line. Several distinct. Phase Diagram Vertical Line.
From galvinconanstuart.blogspot.com
In This Phase Diagram For Water Indicate The Direction That The Solid Phase Diagram Vertical Line Several distinct lines separate these phases. It’s called the phase line. Solid, liquid, gas, and a supercritical fluid. For most compounds, this line has a small positive slope, as shown in the. To be able to identify the triple point, the critical point, and four regions: 13.3 will describe some interesting ternary systems. This simple diagram tells you roughly how. Phase Diagram Vertical Line.
From www.learnmetallurgy.com
Binary Diagrams Phase Diagrams Physical Metallurgy Phase Diagram Vertical Line This section discusses some common kinds of binary systems, and sec. This simple diagram tells you roughly how the system behaves. Solid, liquid, gas, and a supercritical fluid. For most compounds, this line has a small positive slope, as shown in the. These lines are called lines of equilibrium or phase boundaries and represent the equilibrium between the phases. 13.3. Phase Diagram Vertical Line.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram Phase Diagram Vertical Line Several distinct lines separate these phases. This simple diagram tells you roughly how the system behaves. For most compounds, this line has a small positive slope, as shown in the. The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. Solid, liquid, gas, and a supercritical fluid. 13.3 will. Phase Diagram Vertical Line.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram Phase Diagram Vertical Line The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. The phase line captures exactly the information we. To be able to identify the triple point, the critical point, and four regions: It’s called the phase line. Solid, liquid, gas, and a supercritical fluid. Several distinct lines separate these. Phase Diagram Vertical Line.
From www.ck12.org
Phase Diagrams CK12 Foundation Phase Diagram Vertical Line The bd line is almost vertical because the melting point of a solid is not very sensitive to changes in pressure. To be able to identify the triple point, the critical point, and four regions: It’s called the phase line. For most compounds, this line has a small positive slope, as shown in the. 13.3 will describe some interesting ternary. Phase Diagram Vertical Line.
From chem.libretexts.org
13.2 Phase Diagrams Binary Systems Chemistry LibreTexts Phase Diagram Vertical Line For most compounds, this line has a small positive slope, as shown in the. It’s called the phase line. Solid, liquid, gas, and a supercritical fluid. The phase line captures exactly the information we. 13.3 will describe some interesting ternary systems. Several distinct lines separate these phases. This section discusses some common kinds of binary systems, and sec. This simple. Phase Diagram Vertical Line.