Why Are Double Bonds Unstable . While conjugated dienes are energetically more stable than isolated double bonds. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In double bonds, there is also overlap between pi orbitals. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The chemistry of cumulated double bonds can be explored in. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. Cumulated double bonds are unstable. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. In general, in a single bond, there is involvement of sigma bonds only.
from slidetodoc.com
While conjugated dienes are energetically more stable than isolated double bonds. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. In double bonds, there is also overlap between pi orbitals. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In general, in a single bond, there is involvement of sigma bonds only. The chemistry of cumulated double bonds can be explored in. Cumulated double bonds are unstable. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the.
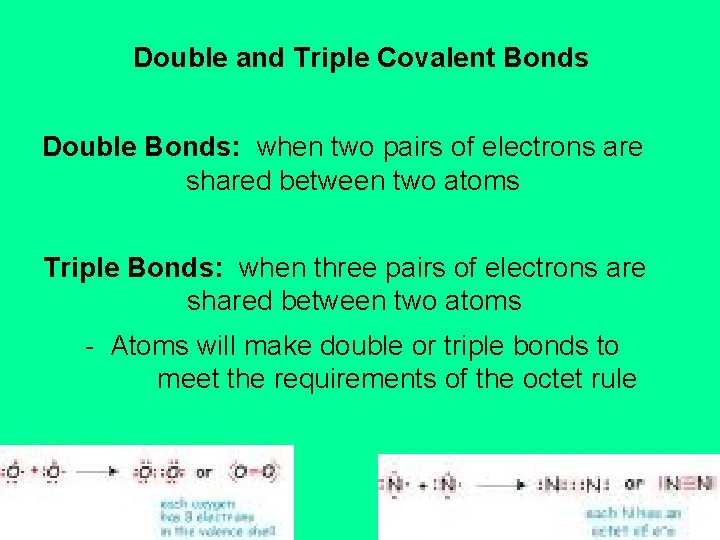
Covalent Bonding Covalent Bond a bond where atoms
Why Are Double Bonds Unstable There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. In general, in a single bond, there is involvement of sigma bonds only. Cumulated double bonds are unstable. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In double bonds, there is also overlap between pi orbitals. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. While conjugated dienes are energetically more stable than isolated double bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The chemistry of cumulated double bonds can be explored in.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Why Are Double Bonds Unstable While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma bonds only. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. The chemistry of cumulated double bonds can be explored in. The reason. Why Are Double Bonds Unstable.
From www.animalia-life.club
Double Bond Why Are Double Bonds Unstable The chemistry of cumulated double bonds can be explored in. In double bonds, there is also overlap between pi orbitals. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. In general, in a single bond, there is involvement of sigma bonds only. Hyperconjugation is a. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Molecular Geometries and Bonding Theories PowerPoint Presentation Why Are Double Bonds Unstable The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. While conjugated dienes are energetically more stable than isolated double bonds. In chemistry,. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT A Gentle Introduction to (or review of) Fundamentals of Chemistry Why Are Double Bonds Unstable In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. While conjugated dienes are energetically more stable than isolated double bonds. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. In double bonds, there is. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Covalent bonding PowerPoint Presentation, free download ID9726629 Why Are Double Bonds Unstable In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The chemistry of cumulated double bonds can be explored in. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. There are a number of important points to keep in mind when considering the. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Nomenclature and Chemical Bonding PowerPoint Presentation, free Why Are Double Bonds Unstable In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. Cumulated double bonds are unstable. While conjugated dienes are energetically more stable. Why Are Double Bonds Unstable.
From www.breakingatom.com
Double bond Definition Why Are Double Bonds Unstable The chemistry of cumulated double bonds can be explored in. In general, in a single bond, there is involvement of sigma bonds only. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. In my organic chemistry book it says that the formation of cyclic hemiacetals. Why Are Double Bonds Unstable.
From www.animalia-life.club
Double Covalent Bond Why Are Double Bonds Unstable In general, in a single bond, there is involvement of sigma bonds only. Cumulated double bonds are unstable. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. The chemistry. Why Are Double Bonds Unstable.
From ar.inspiredpencil.com
Double Bond Lewis Structure Why Are Double Bonds Unstable Cumulated double bonds are unstable. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. While conjugated dienes are energetically more stable than isolated double bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond.. Why Are Double Bonds Unstable.
From www.numerade.com
SOLVEDWhat does a double bond represent? Give two examples of Why Are Double Bonds Unstable The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In general, in a single bond, there is involvement of sigma bonds only. The chemistry of cumulated double bonds can be. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID4132371 Why Are Double Bonds Unstable Cumulated double bonds are unstable. While conjugated dienes are energetically more stable than isolated double bonds. The chemistry of cumulated double bonds can be explored in. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. There are a number of important points to keep in mind when considering the. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation Why Are Double Bonds Unstable In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The chemistry of cumulated double bonds can be explored in. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. While conjugated dienes are energetically more stable. Why Are Double Bonds Unstable.
From chemdictionary.org
Double Covalent Bond Facts, Definition, History & Examples Why Are Double Bonds Unstable While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma bonds only. In double bonds, there is also overlap between pi orbitals. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. In chemistry, a double bond is. Why Are Double Bonds Unstable.
From slidetodoc.com
Covalent Bonding Covalent Bond a bond where atoms Why Are Double Bonds Unstable Cumulated double bonds are unstable. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. In general, in a single bond, there is involvement of sigma bonds only. There are a number of important points to keep in mind when considering the effects of double bonds on. Why Are Double Bonds Unstable.
From www.animalia-life.club
Double Bond Why Are Double Bonds Unstable In double bonds, there is also overlap between pi orbitals. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. In general, in a single bond, there is involvement of sigma bonds only. Cumulated double bonds are unstable. There are a number of important points to keep. Why Are Double Bonds Unstable.
From www.reddit.com
Why does C formed if its more unstable then B? Why is C unstable? Is it Why Are Double Bonds Unstable Cumulated double bonds are unstable. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Double Bond C 2 H 4 PowerPoint Presentation, free download ID Why Are Double Bonds Unstable In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Cumulated double bonds are unstable. In double bonds, there is also overlap between pi orbitals. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule. Why Are Double Bonds Unstable.
From scienceinfo.com
Carbocation Stability Definition, Factors, Order Why Are Double Bonds Unstable Cumulated double bonds are unstable. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma bonds only. In chemistry, a double bond is a covalent. Why Are Double Bonds Unstable.
From www.animalia-life.club
Double Bond Why Are Double Bonds Unstable In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. In general, in a single bond, there is involvement of sigma bonds. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Chapter 22 Chemical Bonding PowerPoint Presentation, free Why Are Double Bonds Unstable In double bonds, there is also overlap between pi orbitals. While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma bonds only. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. There are a. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Bioquímica I Química Biológica I PowerPoint Presentation, free Why Are Double Bonds Unstable While conjugated dienes are energetically more stable than isolated double bonds. In double bonds, there is also overlap between pi orbitals. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. Cumulated double bonds are unstable. Hyperconjugation is a reasonable way to explain why more highly substituted. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID89333 Why Are Double Bonds Unstable In general, in a single bond, there is involvement of sigma bonds only. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. The chemistry of cumulated double bonds can be explored in. In chemistry, a double bond is a covalent bond between two atoms involving four. Why Are Double Bonds Unstable.
From jackwestin.com
Multiple Bonding Covalent Bonds MCAT Content Why Are Double Bonds Unstable In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. Cumulated double bonds are unstable. In double bonds, there is also overlap between pi orbitals. While conjugated dienes are energetically more stable than isolated double bonds. There are a number of important points to keep in mind when considering the. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Chapter 8 lesson 1 Electrons and energy levels PowerPoint Why Are Double Bonds Unstable Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. The. Why Are Double Bonds Unstable.
From study.com
Double Bond Overview, Definition & Examples Lesson Why Are Double Bonds Unstable In double bonds, there is also overlap between pi orbitals. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In general, in a single bond, there is involvement of sigma bonds only. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two. Why Are Double Bonds Unstable.
From www.showme.com
Double Bonds Science, Chemistry, Chemical Bonds ShowMe Why Are Double Bonds Unstable In double bonds, there is also overlap between pi orbitals. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. Hyperconjugation is a reasonable way to. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT Basic Concepts of Chemical Bonding PowerPoint Presentation, free Why Are Double Bonds Unstable In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. There are a number of important points to keep in mind when considering. Why Are Double Bonds Unstable.
From slidetodoc.com
Notes 3 Chemical Bonds Isotopes Ions Bonding properties Why Are Double Bonds Unstable While conjugated dienes are energetically more stable than isolated double bonds. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. Cumulated double bonds are unstable. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. In chemistry, a double bond. Why Are Double Bonds Unstable.
From www.sliderbase.com
Covalent Bonding (Molecules) Presentation Chemistry Why Are Double Bonds Unstable Cumulated double bonds are unstable. In general, in a single bond, there is involvement of sigma bonds only. In double bonds, there is also overlap between pi orbitals. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. The reason is that double bonds, especially when discussing carbon, are reactive. Why Are Double Bonds Unstable.
From definitionklw.blogspot.com
Double Bond Definition Chemistry DEFINITION KLW Why Are Double Bonds Unstable The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. Cumulated double bonds are unstable. The chemistry of cumulated double bonds can be explored in. While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma. Why Are Double Bonds Unstable.
From chem.libretexts.org
9.6 Multiple Bonds Chemistry LibreTexts Why Are Double Bonds Unstable The chemistry of cumulated double bonds can be explored in. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. While conjugated dienes are energetically more stable than isolated double bonds. Cumulated double bonds are unstable. In double bonds, there is also overlap between pi orbitals. Hyperconjugation is a reasonable. Why Are Double Bonds Unstable.
From asiasupergrid.com
What Is A Double Bond Example Why Are Double Bonds Unstable In general, in a single bond, there is involvement of sigma bonds only. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. While conjugated dienes are energetically more stable than isolated double bonds. There are a number of important points to keep in mind when considering. Why Are Double Bonds Unstable.
From www.youtube.com
Examples of Double Covalent Bonds YouTube Why Are Double Bonds Unstable In general, in a single bond, there is involvement of sigma bonds only. In my organic chemistry book it says that the formation of cyclic hemiacetals is energetically favorable in comparison to the. Cumulated double bonds are unstable. Hyperconjugation is a reasonable way to explain why more highly substituted double bonds are more stable. There are a number of important. Why Are Double Bonds Unstable.
From www.slideserve.com
PPT CHEMICAL BONDING PowerPoint Presentation, free download ID5688106 Why Are Double Bonds Unstable There are a number of important points to keep in mind when considering the effects of double bonds on a molecule and its properties. While conjugated dienes are energetically more stable than isolated double bonds. In general, in a single bond, there is involvement of sigma bonds only. The reason is that double bonds, especially when discussing carbon, are reactive. Why Are Double Bonds Unstable.
From www.chemistryworld.com
Unstable silicon double bonds tamed in polymers made on metals Why Are Double Bonds Unstable While conjugated dienes are energetically more stable than isolated double bonds. The reason is that double bonds, especially when discussing carbon, are reactive is that breaking one double bond to make two single bonds. In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. In general,. Why Are Double Bonds Unstable.