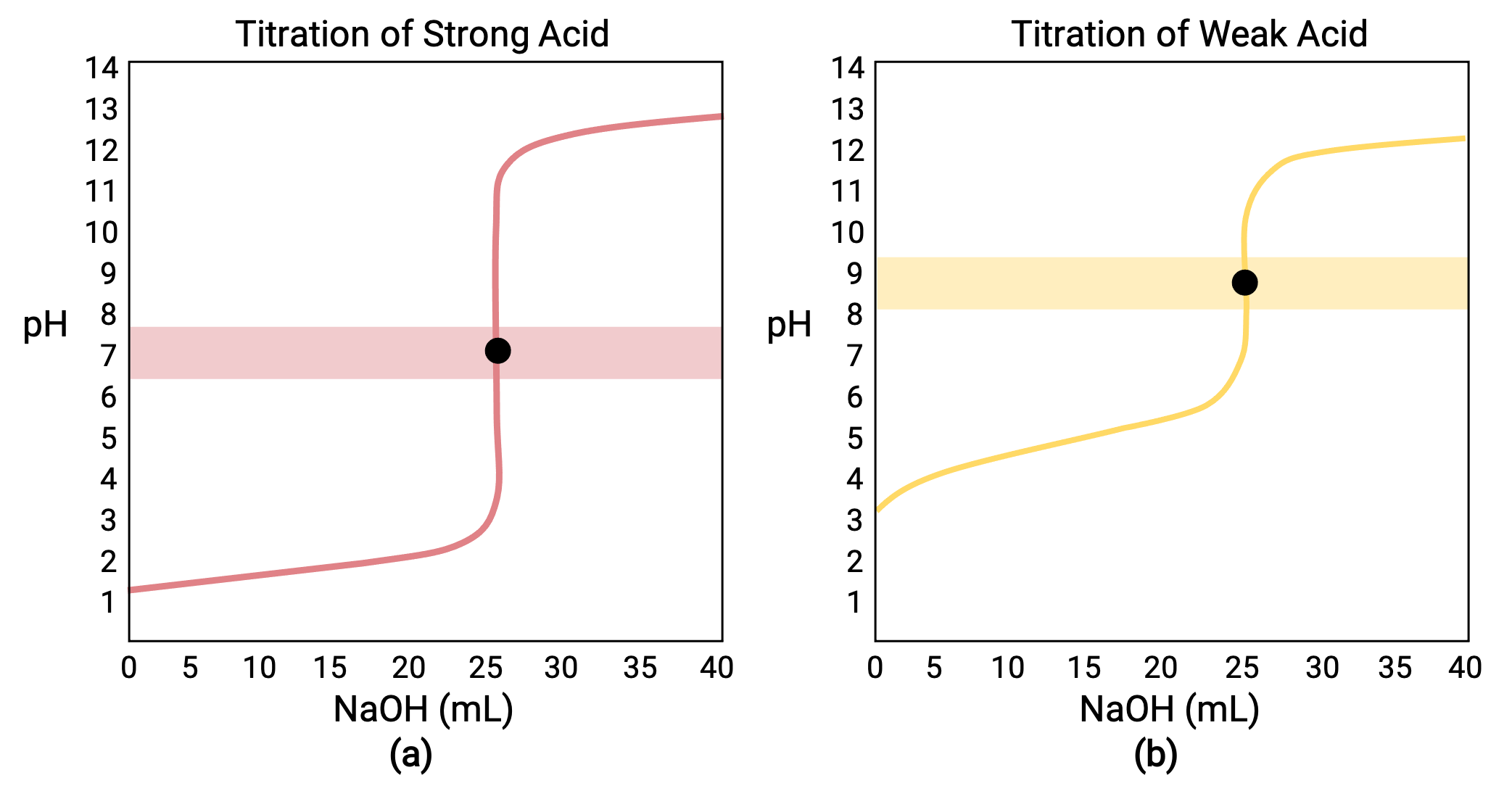
Equivalence Point Of Titration Concentrations . The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7.
from joimexzcj.blob.core.windows.net
If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7.
Ph Titration Definition at Michele Esposito blog
Equivalence Point Of Titration Concentrations The equivalence point of a titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. Equivalence points are not always ph 7. The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which. Equivalence Point Of Titration Concentrations.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If the end point coincides with the addition of the exact chemical equivalence, it is called. Equivalence Point Of Titration Concentrations.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Equivalence Point Of Titration Concentrations The equivalence point of a titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end. Equivalence Point Of Titration Concentrations.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Equivalence Point Of Titration Concentrations The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end point. Equivalence Point Of Titration Concentrations.
From general.chemistrysteps.com
General Chemistry Archives Page 8 of 18 Chemistry Steps Equivalence Point Of Titration Concentrations Equivalence points are not always ph 7. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which. Equivalence Point Of Titration Concentrations.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Equivalence Point Of Titration Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are not always ph 7. The equivalence point of a titration. If the end point coincides with. Equivalence Point Of Titration Concentrations.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Equivalence Point Of Titration Concentrations Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. If the end. Equivalence Point Of Titration Concentrations.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and. Equivalence Point Of Titration Concentrations.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If the end point coincides with the addition of. Equivalence Point Of Titration Concentrations.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Equivalence Point Of Titration Concentrations Equivalence points are not always ph 7. The equivalence point of a titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. If the end point coincides with. Equivalence Point Of Titration Concentrations.
From klaakyfwn.blob.core.windows.net
Dynamic Equivalence Point Titration at Sonja Hogan blog Equivalence Point Of Titration Concentrations The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end point. Equivalence Point Of Titration Concentrations.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Equivalence Point Of Titration Concentrations The equivalence point of a titration. Equivalence points are not always ph 7. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a. Equivalence Point Of Titration Concentrations.
From exojtabwh.blob.core.windows.net
Titration Diprotic Acid at Amy Long blog Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. Equivalence points are not always ph 7. As more base is. Equivalence Point Of Titration Concentrations.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of. Equivalence Point Of Titration Concentrations.
From exyjcpegf.blob.core.windows.net
Titration And Equivalence Point at Bill Hubbard blog Equivalence Point Of Titration Concentrations The equivalence point of a titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. As more base is added and the titration reaches the halfway. Equivalence Point Of Titration Concentrations.
From exyjcpegf.blob.core.windows.net
Titration And Equivalence Point at Bill Hubbard blog Equivalence Point Of Titration Concentrations Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at. Equivalence Point Of Titration Concentrations.
From chemistry.stackexchange.com
Why does the pH before the equivalence point of a titration depend on Equivalence Point Of Titration Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Equivalence points are not always ph 7. If the end point coincides with the addition of the exact chemical. Equivalence Point Of Titration Concentrations.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. If the end point coincides with the addition of the. Equivalence Point Of Titration Concentrations.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated. Equivalence Point Of Titration Concentrations.
From ceedliji.blob.core.windows.net
Titration Curve Analysis at Vera Claar blog Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant. Equivalence Point Of Titration Concentrations.
From exohkacwb.blob.core.windows.net
End Point Of Titration Moles at Melvin Jolly blog Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Equivalence points are not always ph 7. If the end point coincides with the addition of the. Equivalence Point Of Titration Concentrations.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If the end point coincides with the addition of the exact chemical equivalence, it is called. Equivalence Point Of Titration Concentrations.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog Equivalence Point Of Titration Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7. The equivalence point of. Equivalence Point Of Titration Concentrations.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Equivalence Point Of Titration Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. As more base is added and the titration reaches the halfway point, the ph becomes equal to the. Equivalence Point Of Titration Concentrations.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Equivalence Point Of Titration Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Calculate ph at the equivalence point of formic acid titration. Equivalence Point Of Titration Concentrations.
From saylordotorg.github.io
AcidBase Titrations Equivalence Point Of Titration Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are not always ph 7. If the end point coincides with the addition of the exact chemical. Equivalence Point Of Titration Concentrations.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Equivalence Point Of Titration Concentrations The equivalence point of a titration. Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. If the end. Equivalence Point Of Titration Concentrations.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. As more base is added and the titration reaches the halfway point, the ph becomes equal to. Equivalence Point Of Titration Concentrations.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point Of Titration Concentrations Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration. Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of. Equivalence Point Of Titration Concentrations.
From joimexzcj.blob.core.windows.net
Ph Titration Definition at Michele Esposito blog Equivalence Point Of Titration Concentrations As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. Calculate ph at the equivalence point of formic. Equivalence Point Of Titration Concentrations.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Equivalence Point Of Titration Concentrations The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Equivalence points are not always ph 7. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. The equivalence point of a titration. Calculate ph at. Equivalence Point Of Titration Concentrations.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration. As more base is added and the titration reaches the halfway point, the ph becomes equal to the pk a of the acid,. Equivalence points are not always ph 7. The equivalence. Equivalence Point Of Titration Concentrations.
From solvedlib.com
What is a titration? What is the equivalence point o… SolvedLib Equivalence Point Of Titration Concentrations The equivalence point of a titration. Equivalence points are not always ph 7. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. As more base is. Equivalence Point Of Titration Concentrations.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Equivalence Point Of Titration Concentrations The equivalence point of a titration. If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The equivalence point of a titration is the point at which 'chemically. Equivalence Point Of Titration Concentrations.
From klaakyfwn.blob.core.windows.net
Dynamic Equivalence Point Titration at Sonja Hogan blog Equivalence Point Of Titration Concentrations If the end point coincides with the addition of the exact chemical equivalence, it is called the equivalence point or stoichiometric or. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration. Calculate ph at the equivalence point of formic acid titration with. Equivalence Point Of Titration Concentrations.