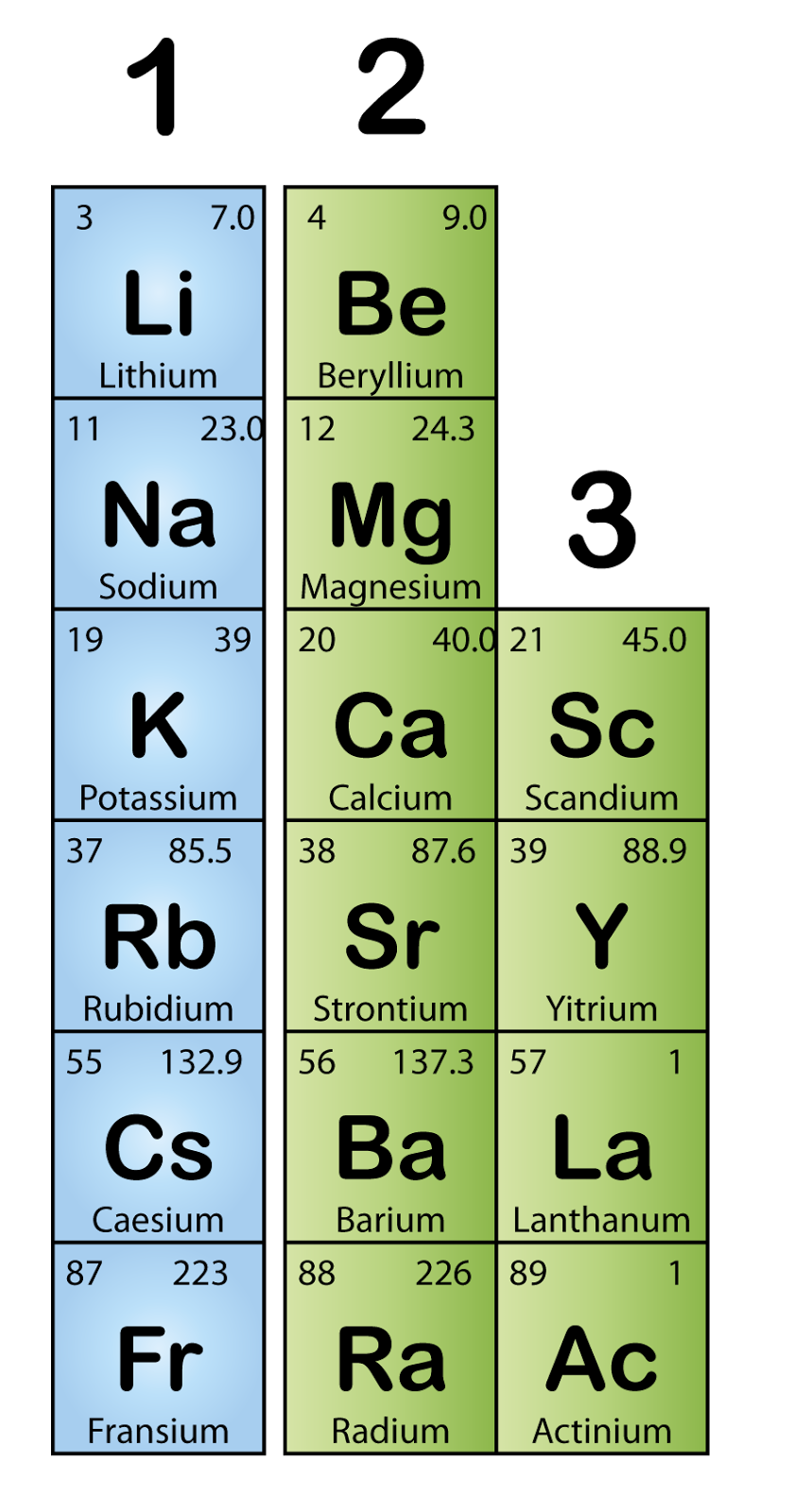
Characteristics Of Alkali Metal . This gives them the largest atomic radii of the elements in their respective periods. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. Elements in the first column of the periodic table. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals are on. The key characteristic these elements share in common is that they all have one. Alkali metals are among the most reactive elements on earth as they. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals tend to form +1 cations.
from m20131000606.blogspot.com
Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. This gives them the largest atomic radii of the elements in their respective periods. The alkali metals are on. Elements in the first column of the periodic table. Alkali metals have one electron in their outer shell, which is loosely bound. Alkali metals are among the most reactive elements on earth as they. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals tend to form +1 cations. The key characteristic these elements share in common is that they all have one.
Chemistry Group 1 Elements Alkali Metals
Characteristics Of Alkali Metal Alkali metals are among the most reactive elements on earth as they. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals are among the most reactive elements on earth as they. The alkali metals are on. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. This gives them the largest atomic radii of the elements in their respective periods. The key characteristic these elements share in common is that they all have one. Alkali metals have one electron in their outer shell, which is loosely bound. Elements in the first column of the periodic table. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals tend to form +1 cations.
From armsingle10.pythonanywhere.com
Brilliant Bbc Bitesize Periodic Table Magnesium Chloride Reaction With Characteristics Of Alkali Metal Alkali metals are among the most reactive elements on earth as they. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Alkali metals have one electron in their outer shell,. Characteristics Of Alkali Metal.
From www.youtube.com
ALKALI METALS YouTube Characteristics Of Alkali Metal Alkali metals are among the most reactive elements on earth as they. The alkali metals are on. The alkali metals are the elements located in group ia of the periodic table (the first column). This gives them the largest atomic radii of the elements in their respective periods. Understand the electronic configurations, ionization enthalpy, radii and other properties of group. Characteristics Of Alkali Metal.
From byjus.com
Alkaline Earth Metals Occurrence and Extraction,Physical Properties Characteristics Of Alkali Metal The key characteristic these elements share in common is that they all have one. Alkali metals are among the most reactive elements on earth as they. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are on. The alkali metals tend to form +1 cations. The alkali metals are the elements located in. Characteristics Of Alkali Metal.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table Characteristics Of Alkali Metal The alkali metals tend to form +1 cations. Elements in the first column of the periodic table. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The key characteristic these elements share in common is that they all have one. The alkali. Characteristics Of Alkali Metal.
From byjus.com
Alkaline Earth Metals General Characteristics of Oxides, Hallides Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to. Characteristics Of Alkali Metal.
From en.sdppal.cn
Aluminummagnesiummanganese metal plateShandong Taihui New Materials Characteristics Of Alkali Metal Alkali metals are among the most reactive elements on earth as they. Elements in the first column of the periodic table. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to. Characteristics Of Alkali Metal.
From www.youtube.com
Occurrence of ALKALI & ALKALINE Earth Metals SBlock Elements F Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are the elements located in group ia of the periodic table (the first column). Alkali metals are among the most reactive elements on earth as they. Elements in the first column of the periodic table. Cation formation is favored by the relatively low ionization. Characteristics Of Alkali Metal.
From www.sliderbase.com
Element Classes Presentation Chemistry Characteristics Of Alkali Metal The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are on. The key characteristic these elements share in common is that they. Characteristics Of Alkali Metal.
From www.slideserve.com
PPT THE PERIODIC TABLE PowerPoint Presentation, free download ID Characteristics Of Alkali Metal The alkali metals are on. The key characteristic these elements share in common is that they all have one. The alkali metals tend to form +1 cations. This gives them the largest atomic radii of the elements in their respective periods. Elements in the first column of the periodic table. The alkali metals are the elements located in group ia. Characteristics Of Alkali Metal.
From www.slideshare.net
GENERAL CHARACTERISTICS OF THE COMPOUNDS OF ALKALI METALS Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. This gives them the largest atomic radii of the elements in their respective periods. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are on. The alkali metals. Characteristics Of Alkali Metal.
From ravennewsrogers.blogspot.com
Describe the Properties of Alkali Metals Characteristics Of Alkali Metal The alkali metals tend to form +1 cations. The alkali metals are the elements located in group ia of the periodic table (the first column). The key characteristic these elements share in common is that they all have one. Elements in the first column of the periodic table. The alkali metals are on. Understand the electronic configurations, ionization enthalpy, radii. Characteristics Of Alkali Metal.
From www.revimage.org
Difference Between Alkali And Alkaline Earth Metals The Earth Images Characteristics Of Alkali Metal Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are on. The alkali metals are the elements located in group ia of the periodic table (the first column). Elements in the first column of the periodic table. This gives them the. Characteristics Of Alkali Metal.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) Characteristics Of Alkali Metal The alkali metals are on. Elements in the first column of the periodic table. Alkali metals are among the most reactive elements on earth as they. Alkali metals have one electron in their outer shell, which is loosely bound. This gives them the largest atomic radii of the elements in their respective periods. Cation formation is favored by the relatively. Characteristics Of Alkali Metal.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 Teaching Resources Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are the elements located in group ia of the periodic table (the first column). Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are all. Characteristics Of Alkali Metal.
From www.sliderbase.com
Group 1&2 Presentation Chemistry Characteristics Of Alkali Metal Elements in the first column of the periodic table. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals are on. Alkali metals are among the most reactive elements on earth as they. The alkali metals are the elements located. Characteristics Of Alkali Metal.
From www.pinterest.com
Characteristics of alkali metals Alkali metal, Metal, Middle school Characteristics Of Alkali Metal The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals are on. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals. Characteristics Of Alkali Metal.
From elchoroukhost.net
Properties Of Alkali Metals On The Periodic Table Elcho Table Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. This gives them the largest atomic radii of the elements in their respective periods. The key characteristic these elements share in common. Characteristics Of Alkali Metal.
From www.revimage.org
The Activity Of Alkaline Earth Metals As Reducing Agent The Earth Characteristics Of Alkali Metal Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. This gives them the largest atomic radii of the elements in their respective periods. Alkali metals have one electron in their. Characteristics Of Alkali Metal.
From study.com
Alkali Metals Definition, Properties & Characteristics Lesson Characteristics Of Alkali Metal Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. This gives them the largest atomic radii of. Characteristics Of Alkali Metal.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals Characteristics Of Alkali Metal Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. This gives them the largest atomic radii of the elements in their respective periods. Alkali metals have one electron in their outer shell, which is loosely bound. Understand the electronic configurations, ionization enthalpy, radii and. Characteristics Of Alkali Metal.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free Characteristics Of Alkali Metal The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals tend to form +1 cations. Alkali metals are among the most reactive elements on earth as they. Elements in the first column of the periodic table. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali.. Characteristics Of Alkali Metal.
From utedzz.blogspot.com
Periodic Table Alkali Metals Periodic Table Timeline Characteristics Of Alkali Metal This gives them the largest atomic radii of the elements in their respective periods. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals are the elements located in group ia of the periodic table (the first column). Cation formation. Characteristics Of Alkali Metal.
From www.slideshare.net
ChemistryGeneral Characteristics Of Alkali Metals Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. The key characteristic these elements share in common is that they all have one. The alkali metals are on. This gives them the largest atomic radii of the elements in their respective periods. Elements in the first column of the periodic table. Understand the electronic configurations, ionization. Characteristics Of Alkali Metal.
From www.revimage.org
Similarities And Differences Between Alkali Metals Alkaline Earth The Characteristics Of Alkali Metal The key characteristic these elements share in common is that they all have one. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. This gives them the largest atomic. Characteristics Of Alkali Metal.
From atomicradiichart.z1.web.core.windows.net
order of atomic radius of group 13 elements Boron family elements group Characteristics Of Alkali Metal Elements in the first column of the periodic table. The alkali metals are on. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron. Characteristics Of Alkali Metal.
From en.sdppal.cn
Aluminummagnesiummanganese metal plateShandong Taihui New Materials Characteristics Of Alkali Metal Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Alkali metals are among the most reactive elements on earth as they. The alkali metals tend to form +1 cations. The. Characteristics Of Alkali Metal.
From byjus.com
Alkali Metals Chemical and Physical Properties of Alkali Metals Characteristics Of Alkali Metal This gives them the largest atomic radii of the elements in their respective periods. Elements in the first column of the periodic table. The alkali metals are the elements located in group ia of the periodic table (the first column). The alkali metals are on. The alkali metals tend to form +1 cations. Alkali metals have one electron in their. Characteristics Of Alkali Metal.
From cabinet.matttroy.net
Alkaline Earth Metals Periodic Table Definition Matttroy Characteristics Of Alkali Metal The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The alkali metals tend to form +1 cations. Alkali metals have one electron in their outer shell, which is loosely bound. Understand the electronic configurations, ionization enthalpy, radii and other properties of group. Characteristics Of Alkali Metal.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Characteristics Of Alkali Metal The key characteristic these elements share in common is that they all have one. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals tend to form +1 cations. Alkali metals are among the most reactive elements on earth as they. Alkali. Characteristics Of Alkali Metal.
From sites.google.com
C2 The Periodic Table Kingshill Science Characteristics Of Alkali Metal The alkali metals are the elements located in group ia of the periodic table (the first column). The key characteristic these elements share in common is that they all have one. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. The alkali metals are. Characteristics Of Alkali Metal.
From meixi-mgo.com
Use and synthesis of nano magnesium hydroxide Magnesia Supplier Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are on. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. Elements. Characteristics Of Alkali Metal.
From www.revimage.org
Where Are Alkaline Earth Metals Found On The Periodic Table Brainly Characteristics Of Alkali Metal Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali. The key characteristic these elements share in common is that they all have one. This gives them the largest atomic radii. Characteristics Of Alkali Metal.
From reviewhomedecor.co
Alkaline Earth Metal Periodic Table Facts Review Home Decor Characteristics Of Alkali Metal Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are on. This gives them the largest atomic radii of the elements in their respective periods. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. The. Characteristics Of Alkali Metal.
From www.youtube.com
Properties of Alkali and Alkaline Earth Metals Hydroxides, Chemistry Characteristics Of Alkali Metal The key characteristic these elements share in common is that they all have one. The alkali metals tend to form +1 cations. Alkali metals have one electron in their outer shell, which is loosely bound. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with. Characteristics Of Alkali Metal.
From www.askiitians.com
Alkaline Earth Metals Study Material for IIT JEE askIITians Characteristics Of Alkali Metal This gives them the largest atomic radii of the elements in their respective periods. The alkali metals are on. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. Understand the electronic configurations, ionization enthalpy, radii and other properties of group one alkali.. Characteristics Of Alkali Metal.