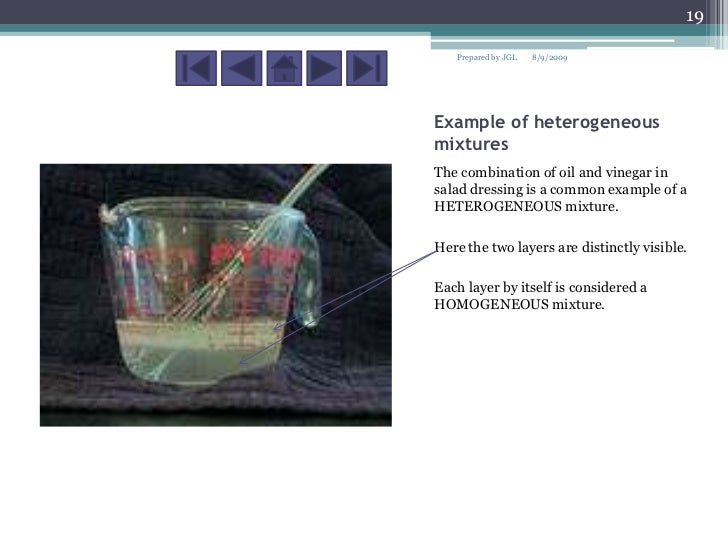
Vinegar And Oil Heterogeneous Mixture . A heterogeneous mixture consists of two or more phases. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the properties and examples of heterogeneous. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. When oil and water are combined, they do not mix evenly, but instead form two separate. The answer lies in its composition.
from www.slideshare.net
Learn the properties and examples of heterogeneous. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. When oil and water are combined, they do not mix evenly, but instead form two separate. The answer lies in its composition. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. A heterogeneous mixture consists of two or more phases. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone.
Mixtures And Their Separations
Vinegar And Oil Heterogeneous Mixture You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. A heterogeneous mixture consists of two or more phases. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn the properties and examples of heterogeneous. The answer lies in its composition. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. When oil and water are combined, they do not mix evenly, but instead form two separate.
From www.slideshare.net
Mixtures And Their Separations Vinegar And Oil Heterogeneous Mixture Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn why vinegar is a homogeneous mixture of water and acetic acid, and. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Concept Data Theory Metal Nonmetal Molar Mass Avogadros Number Theory Vinegar And Oil Heterogeneous Mixture Learn the properties and examples of heterogeneous. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. A heterogeneous mixture consists of two or more phases. When oil and water are combined, they do not mix evenly, but instead form two. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Matter Elements, Compounds, and Mixtures ppt download Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn the properties and examples of heterogeneous. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. The answer lies in its composition. Learn the definitions and examples of homogeneous. Vinegar And Oil Heterogeneous Mixture.
From www.slideserve.com
PPT Mixtures PowerPoint Presentation, free download ID5353398 Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. A heterogeneous mixture consists of two or more phases. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. The answer. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Water and Aqueous Systems ppt download Vinegar And Oil Heterogeneous Mixture You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. Learn the properties and examples of heterogeneous. The answer lies in its composition.. Vinegar And Oil Heterogeneous Mixture.
From www.teachoo.com
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo Vinegar And Oil Heterogeneous Mixture Learn the properties and examples of heterogeneous. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn why vinegar is. Vinegar And Oil Heterogeneous Mixture.
From sciencenotes.org
What Is a Heterogeneous Mixture? Definition and Examples Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. Learn the properties and examples of heterogeneous. When oil and water are combined, they do not mix evenly, but instead form two. Vinegar And Oil Heterogeneous Mixture.
From gioevykeo.blob.core.windows.net
Salad Dressing Heterogeneous Mixture at Harold Gonzales blog Vinegar And Oil Heterogeneous Mixture A heterogeneous mixture consists of two or more phases. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Vinegar is a solution of acetic acid and water, along with trace amounts. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Heterogeneous Mixture ppt download Vinegar And Oil Heterogeneous Mixture When oil and water are combined, they do not mix evenly, but instead form two separate. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. The answer lies in its composition. Learn the properties and examples of heterogeneous. Learn why vinegar is a homogeneous mixture of water and acetic. Vinegar And Oil Heterogeneous Mixture.
From www.sciencephoto.com
Oil and vinegar, 2 of 2 Stock Image C050/4776 Science Photo Library Vinegar And Oil Heterogeneous Mixture Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. The answer lies in its composition. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. When oil and water are combined, they do not mix evenly, but instead form. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Biochemistry. ppt download Vinegar And Oil Heterogeneous Mixture When oil and water are combined, they do not mix evenly, but instead form two separate. Learn the properties and examples of heterogeneous. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Chapter 1 Matter and Change ppt download Vinegar And Oil Heterogeneous Mixture Learn the properties and examples of heterogeneous. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. The answer lies in its composition. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. You can (and should!) shake up a. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Key Ideas What is a heterogeneous mixture? ppt download Vinegar And Oil Heterogeneous Mixture You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the properties and examples of heterogeneous. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn the definitions and. Vinegar And Oil Heterogeneous Mixture.
From hxebkdxil.blob.core.windows.net
Oil And Vinegar Is A Heterogeneous Mixture at Melinda Allen blog Vinegar And Oil Heterogeneous Mixture Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. A heterogeneous mixture consists of two or more phases. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the. Vinegar And Oil Heterogeneous Mixture.
From hxebkdxil.blob.core.windows.net
Oil And Vinegar Is A Heterogeneous Mixture at Melinda Allen blog Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. Learn the properties and examples of heterogeneous. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Mixtures. ppt download Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn why vinegar is a homogeneous mixture. Vinegar And Oil Heterogeneous Mixture.
From www.numerade.com
SOLVED For each of the following mixtures, state whether it is Vinegar And Oil Heterogeneous Mixture Learn the properties and examples of heterogeneous. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. When oil and water are combined,. Vinegar And Oil Heterogeneous Mixture.
From www.slideserve.com
PPT PSc.2.1 OBJECTIVE Understand types, properties and structure of Vinegar And Oil Heterogeneous Mixture When oil and water are combined, they do not mix evenly, but instead form two separate. Learn the properties and examples of heterogeneous. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. The answer lies in its composition. You can (and should!) shake up a vinaigrette to make the mixture appear. Vinegar And Oil Heterogeneous Mixture.
From lilia-blogmontgomery.blogspot.com
Oil and Vinegar Salad Dressing Homogeneous or Heterogeneous Vinegar And Oil Heterogeneous Mixture Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. The answer lies in its composition. A heterogeneous mixture consists of two or more phases. When oil and water are combined, they do not mix evenly, but instead form two separate. You can (and should!) shake up a vinaigrette to. Vinegar And Oil Heterogeneous Mixture.
From foodandfizz.com
Is Salad Dressing A Heterogeneous Or Homogeneous Mixture? Vinegar And Oil Heterogeneous Mixture A heterogeneous mixture consists of two or more phases. Learn the properties and examples of heterogeneous. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. You can (and should!) shake up. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Chemistry ppt download Vinegar And Oil Heterogeneous Mixture A heterogeneous mixture consists of two or more phases. Learn the properties and examples of heterogeneous. When oil and water are combined, they do not mix evenly, but instead form two separate. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn why vinegar is a homogeneous mixture of. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Mr. Mott 10th Grade Chemistry ppt download Vinegar And Oil Heterogeneous Mixture When oil and water are combined, they do not mix evenly, but instead form two separate. The answer lies in its composition. Learn the properties and examples of heterogeneous. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. A heterogeneous mixture consists of two or more phases. You can. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Chapter 15 Classification of matter ppt video online download Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the properties and examples of heterogeneous. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances.. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Chemistry Skills Check 1 ppt download Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the properties and examples of heterogeneous. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. A heterogeneous mixture consists of two or more phases.. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
10.1 Classifying Matter. ppt download Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. A heterogeneous. Vinegar And Oil Heterogeneous Mixture.
From www.numerade.com
Ayala is making salad dressing. She mixes oil and vinegar in a blender Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. When oil and water are combined, they do not mix evenly, but instead form two separate. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its. Vinegar And Oil Heterogeneous Mixture.
From gatherforbread.com
Oil vinegar mixture Gather for Bread Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and. Vinegar And Oil Heterogeneous Mixture.
From study.com
Heterogeneous Mixture Definition, Types & Examples Video & Lesson Vinegar And Oil Heterogeneous Mixture Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. Learn the properties and examples of heterogeneous. A heterogeneous mixture consists of two or more phases. The answer lies in its composition.. Vinegar And Oil Heterogeneous Mixture.
From studiousguy.com
10 Homogeneous Mixture Examples in Daily Life StudiousGuy Vinegar And Oil Heterogeneous Mixture Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn why vinegar is a homogeneous mixture of water and acetic. Vinegar And Oil Heterogeneous Mixture.
From www.numerade.com
SOLVED 'pahelp po please kailangan na wnat S More Activity A Vinegar And Oil Heterogeneous Mixture A heterogeneous mixture consists of two or more phases. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. When oil and water are combined, they do not mix evenly, but instead form two separate. Learn the definitions and examples of. Vinegar And Oil Heterogeneous Mixture.
From mungfali.com
10 Examples Of Heterogeneous Mixture Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. When oil and water are combined, they do not mix evenly, but instead form two separate. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Classification of Matter ppt download Vinegar And Oil Heterogeneous Mixture The answer lies in its composition. A heterogeneous mixture consists of two or more phases. Vinegar is a solution of acetic acid and water, along with trace amounts of other chemicals that give it its. Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. When oil and water are. Vinegar And Oil Heterogeneous Mixture.
From eduforall.us
24 Fact Checked Examples of Heterogenous Mixtures & their Components Vinegar And Oil Heterogeneous Mixture Learn the definitions and examples of homogeneous and heterogeneous mixtures, and how to distinguish them from pure substances. A heterogeneous mixture consists of two or more phases. The answer lies in its composition. When oil and water are combined, they do not mix evenly, but instead form two separate. Vinegar is a solution of acetic acid and water, along with. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Biochemistry The Chemistry of Life Text Chapter ppt download Vinegar And Oil Heterogeneous Mixture When oil and water are combined, they do not mix evenly, but instead form two separate. The answer lies in its composition. A heterogeneous mixture consists of two or more phases. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone.. Vinegar And Oil Heterogeneous Mixture.
From slideplayer.com
Do Now. ppt download Vinegar And Oil Heterogeneous Mixture Learn why vinegar is a homogeneous mixture of water and acetic acid, and how it differs from a heterogeneous mixture. You can (and should!) shake up a vinaigrette to make the mixture appear and taste more combined, but it will always separate into its component parts when left alone. Learn the definitions and examples of homogeneous and heterogeneous mixtures, and. Vinegar And Oil Heterogeneous Mixture.