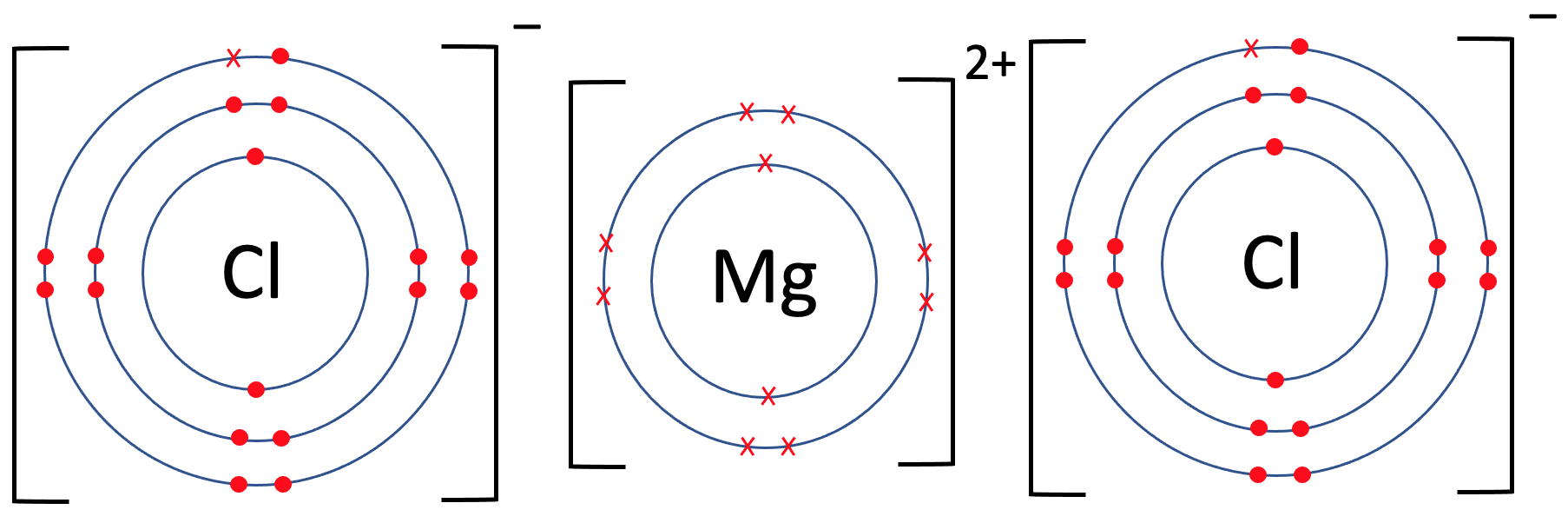
Chlorine Structure And Bonding . This is called covalent bonding. So by sharing electrons through. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. Chlorine is commonly used as antiseptic. To get a full outer shell and form a stable cl 2 molecule. the two chlorine atoms are said to be joined by a covalent bond. in chlorine an electron pair is shared between the two atoms in cl 2. the two chlorine atoms are said to be joined by a covalent bond. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. chlorine is the chemical name of cl. chlorine is in group 7 of the periodic table. The reason that the two chlorine atoms stick together is that the. Two chlorine atoms will each share one electron. Visit byju's to understand the properties,.
from www.elevise.co.uk
the two chlorine atoms are said to be joined by a covalent bond. This is called covalent bonding. So by sharing electrons through. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. chlorine is in group 7 of the periodic table. the two chlorine atoms are said to be joined by a covalent bond. in chlorine an electron pair is shared between the two atoms in cl 2. To get a full outer shell and form a stable cl 2 molecule. The reason that the two chlorine atoms stick together is that the. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine.
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise
Chlorine Structure And Bonding To get a full outer shell and form a stable cl 2 molecule. The reason that the two chlorine atoms stick together is that the. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. the two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. Chlorine is commonly used as antiseptic. Two chlorine atoms will each share one electron. Visit byju's to understand the properties,. This is called covalent bonding. So by sharing electrons through. chlorine is the chemical name of cl. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. To get a full outer shell and form a stable cl 2 molecule. in chlorine an electron pair is shared between the two atoms in cl 2. the two chlorine atoms are said to be joined by a covalent bond.
From schematicdiagramyakuza.z13.web.core.windows.net
Lewis Dot Diagram Chlorine Chlorine Structure And Bonding however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. chlorine is in group 7 of the periodic table. Chlorine is commonly used as antiseptic. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. chlorine has a far larger electronegativity and so pulls. Chlorine Structure And Bonding.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Chlorine Structure And Bonding in chlorine an electron pair is shared between the two atoms in cl 2. To get a full outer shell and form a stable cl 2 molecule. Chlorine is commonly used as antiseptic. chlorine is in group 7 of the periodic table. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely,. Chlorine Structure And Bonding.
From imgbin.com
Lewis Structure Electron Chlorine Diagram Chloride PNG, Clipart, Black Chlorine Structure And Bonding This is called covalent bonding. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. the two chlorine atoms are said to be joined by a covalent bond. chlorine is the. Chlorine Structure And Bonding.
From slideplayer.com
Chemical Bonds Chapter ppt download Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. the two chlorine atoms are said to be joined by a covalent bond. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that the two chlorine atoms stick together is that the shared pair of. Chlorine Structure And Bonding.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Chlorine Structure And Bonding To get a full outer shell and form a stable cl 2 molecule. The reason that the two chlorine atoms stick together is that the. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Chlorine is commonly used as antiseptic. in chlorine an electron pair is shared between the two atoms. Chlorine Structure And Bonding.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. So by sharing electrons through. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. chlorine is the chemical name of cl. Chlorine is commonly used as antiseptic. The reason. Chlorine Structure And Bonding.
From www.youtube.com
Covalent Bonding Chlorine Gas YouTube Chlorine Structure And Bonding chlorine is the chemical name of cl. Visit byju's to understand the properties,. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. The reason that the two chlorine atoms stick together is that the. Two chlorine atoms will each share one electron. however, there are two key. Chlorine Structure And Bonding.
From www.youtube.com
Covalent Bond of Hydrogen , Chlorine and Hydro Chloride Molecule Chlorine Structure And Bonding Visit byju's to understand the properties,. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. To get a full outer shell and form a stable cl 2 molecule. Chlorine is commonly used as antiseptic. the two chlorine atoms are said to be joined by a covalent bond. in chlorine an. Chlorine Structure And Bonding.
From guidediagramterrain.z5.web.core.windows.net
The Lewis Dot Diagram For Ionic Bonding Chlorine Structure And Bonding So by sharing electrons through. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. the two chlorine atoms are said to be joined by a covalent bond. Two chlorine atoms will each share one electron. however, there are two key features with. Chlorine Structure And Bonding.
From egpat.com
Lewis dot structure How to write? Chlorine Structure And Bonding chlorine is in group 7 of the periodic table. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. Chlorine is commonly used as antiseptic. chlorine. Chlorine Structure And Bonding.
From www.vedantu.com
Chemical Bonding and Molecular Structure Class 11 Notes CBSE Chemistry Chlorine Structure And Bonding chlorine is the chemical name of cl. To get a full outer shell and form a stable cl 2 molecule. the two chlorine atoms are said to be joined by a covalent bond. the two chlorine atoms are said to be joined by a covalent bond. Two chlorine atoms will each share one electron. however, there. Chlorine Structure And Bonding.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. chlorine is in group 7 of the periodic table. in chlorine an electron pair is shared between the two atoms in cl 2. So by sharing electrons through. The reason that the two chlorine atoms stick together is that the. This is called covalent. Chlorine Structure And Bonding.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Structure And Bonding The reason that the two chlorine atoms stick together is that the shared pair of electrons is. The reason that the two chlorine atoms stick together is that the. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. Two chlorine atoms will each share one electron. So by sharing electrons through. . Chlorine Structure And Bonding.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Structure And Bonding Two chlorine atoms will each share one electron. Visit byju's to understand the properties,. the two chlorine atoms are said to be joined by a covalent bond. To get a full outer shell and form a stable cl 2 molecule. the two chlorine atoms are said to be joined by a covalent bond. chlorine has a far. Chlorine Structure And Bonding.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Structure And Bonding chlorine is the chemical name of cl. Visit byju's to understand the properties,. Chlorine is commonly used as antiseptic. in chlorine an electron pair is shared between the two atoms in cl 2. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. the two chlorine atoms. Chlorine Structure And Bonding.
From www.vedantu.com
Chlorine Learn Definition, Properties and Facts Chlorine Structure And Bonding Chlorine is commonly used as antiseptic. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. Visit byju's to understand the properties,. So by sharing electrons through. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. The. Chlorine Structure And Bonding.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Structure And Bonding The reason that the two chlorine atoms stick together is that the shared pair of electrons is. in chlorine an electron pair is shared between the two atoms in cl 2. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. chlorine is the chemical name of cl.. Chlorine Structure And Bonding.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.7 Covalent Bonding翰林国际教育 Chlorine Structure And Bonding Chlorine is commonly used as antiseptic. the two chlorine atoms are said to be joined by a covalent bond. This is called covalent bonding. chlorine is in group 7 of the periodic table. in chlorine an electron pair is shared between the two atoms in cl 2. the two chlorine atoms are said to be joined. Chlorine Structure And Bonding.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Structure And Bonding chlorine is the chemical name of cl. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. So by sharing electrons through. Visit byju's to understand the properties,. chlorine is in group 7 of the periodic table. Two chlorine atoms will each share. Chlorine Structure And Bonding.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology Chlorine Structure And Bonding in chlorine an electron pair is shared between the two atoms in cl 2. Two chlorine atoms will each share one electron. chlorine is the chemical name of cl. Chlorine is commonly used as antiseptic. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. however, there are two key. Chlorine Structure And Bonding.
From circuitlistethiops123.z13.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. Two chlorine atoms will each share one electron. To get a full outer shell and form a stable cl 2 molecule. Chlorine is commonly used as antiseptic. Visit byju's to understand the properties,. The reason that the two chlorine atoms stick together is that the. . Chlorine Structure And Bonding.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Structure And Bonding To get a full outer shell and form a stable cl 2 molecule. in chlorine an electron pair is shared between the two atoms in cl 2. chlorine is the chemical name of cl. This is called covalent bonding. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its. Chlorine Structure And Bonding.
From mavink.com
Chlorine Molecule Diagram Chlorine Structure And Bonding chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. the two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the. The reason that the two chlorine atoms stick together. Chlorine Structure And Bonding.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Structure And Bonding chlorine is the chemical name of cl. This is called covalent bonding. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. Chlorine is commonly used as antiseptic. in chlorine an electron pair is shared between the two atoms in cl 2. the two chlorine atoms are said to be. Chlorine Structure And Bonding.
From circuitenginegrave101.z5.web.core.windows.net
Carbon And Chlorine Electron Dot Diagram Chlorine Structure And Bonding Chlorine is commonly used as antiseptic. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. chlorine is the chemical name of cl. To get a full. Chlorine Structure And Bonding.
From secondaryscience4all.wordpress.com
Dot and cross diagrams for simple covalent molecules (I) Secondary Chlorine Structure And Bonding in chlorine an electron pair is shared between the two atoms in cl 2. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. chlorine is the chemical name of cl. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. The reason that. Chlorine Structure And Bonding.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. The reason that the two chlorine atoms stick together is that the. So by sharing electrons through. chlorine is the chemical name of cl. Visit byju's to understand the properties,. This is called covalent bonding. To get a full outer shell and form a stable. Chlorine Structure And Bonding.
From chem.libretexts.org
10.3 Compounds of Chlorine Chemistry LibreTexts Chlorine Structure And Bonding So by sharing electrons through. Chlorine is commonly used as antiseptic. however, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine. This is called covalent bonding. Visit byju's to understand the properties,. chlorine is in group 7 of the periodic table. To get a full outer shell and form a stable cl. Chlorine Structure And Bonding.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. the two chlorine atoms are said to be joined by a covalent bond. chlorine is the chemical name of. Chlorine Structure And Bonding.
From www.youtube.com
Draw the Lewis Structure of KCl (potassium chloride) YouTube Chlorine Structure And Bonding This is called covalent bonding. chlorine is in group 7 of the periodic table. the two chlorine atoms are said to be joined by a covalent bond. the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure,. however, there are two key features with regard to chlorine’s. Chlorine Structure And Bonding.
From exopqejyx.blob.core.windows.net
Chlorine Electron Pairs at Lewis Stallings blog Chlorine Structure And Bonding Visit byju's to understand the properties,. Two chlorine atoms will each share one electron. The reason that the two chlorine atoms stick together is that the. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. Chlorine is commonly used as antiseptic. The reason that. Chlorine Structure And Bonding.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. in chlorine an electron pair is shared between the two atoms in cl 2. This is called covalent bonding. The reason that the two chlorine atoms stick together is that the. Chlorine is commonly used as antiseptic. the two chlorine atoms are said to. Chlorine Structure And Bonding.
From www.youtube.com
Cl2 (Chlorine gas) Molecular Geometry, Bond Angles YouTube Chlorine Structure And Bonding Visit byju's to understand the properties,. So by sharing electrons through. chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and forming a negative ion. The reason that the two chlorine atoms stick together is that the shared pair of electrons is. however, there are two key features. Chlorine Structure And Bonding.
From waterengineers.com.au
Chlorine Dioxide Water Engineers Chlorine Structure And Bonding the two chlorine atoms are said to be joined by a covalent bond. Visit byju's to understand the properties,. The reason that the two chlorine atoms stick together is that the. To get a full outer shell and form a stable cl 2 molecule. in chlorine an electron pair is shared between the two atoms in cl 2.. Chlorine Structure And Bonding.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Structure And Bonding The reason that the two chlorine atoms stick together is that the shared pair of electrons is. chlorine is in group 7 of the periodic table. Visit byju's to understand the properties,. So by sharing electrons through. the two chlorine atoms are said to be joined by a covalent bond. chlorine is the chemical name of cl.. Chlorine Structure And Bonding.