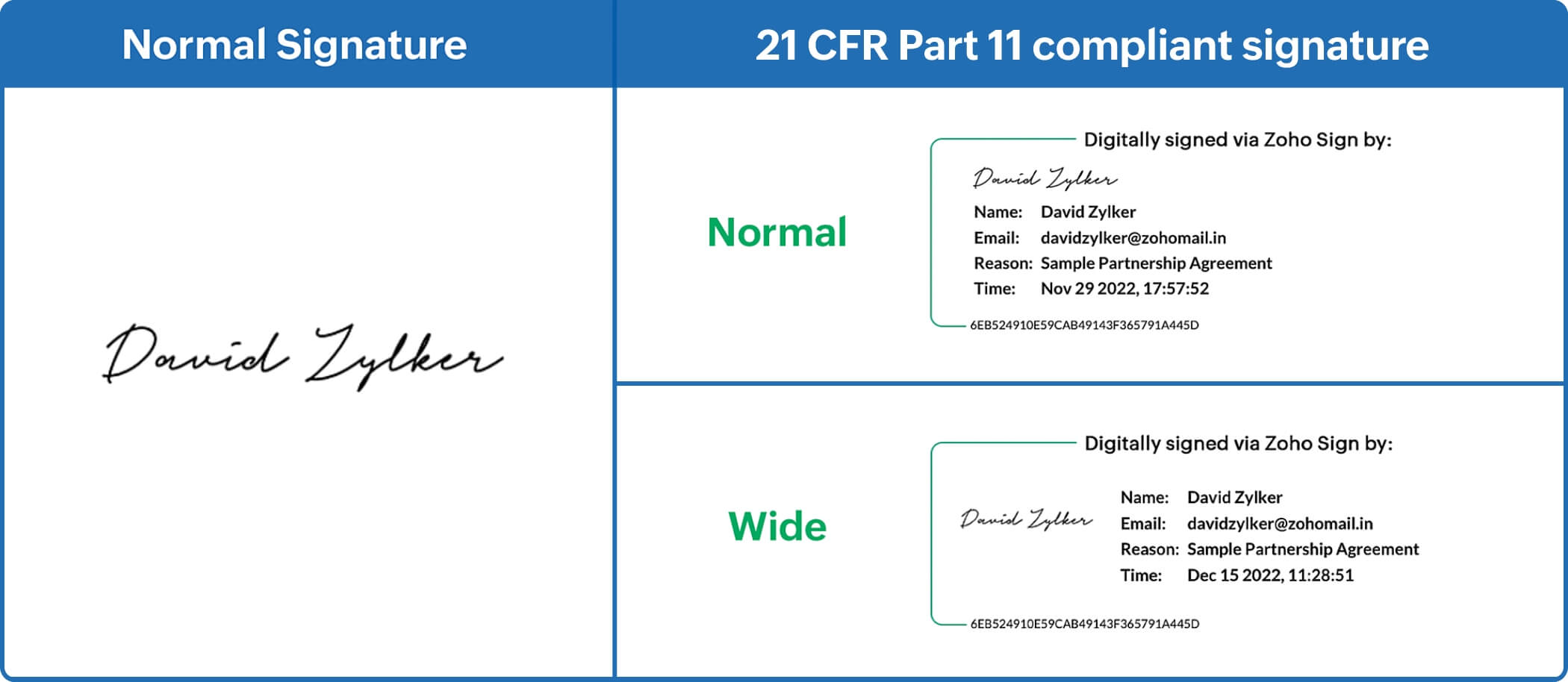
Electronic Signature Policy For Medical Records . We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. Electronic signatures — scope and application. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions.
from www.zoho.com
In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. Electronic signatures — scope and application. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions.
FDA 21 CFR Part 11 compliance with electronic signatures Zoho Sign
Electronic Signature Policy For Medical Records Electronic signatures — scope and application. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. Electronic signatures — scope and application. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions.
From signature-watermark.com
Quantum Innovations emedical records, elaw records, digital Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. Electronic signatures — scope and application. We encourage you to send a complete medical record. Electronic Signature Policy For Medical Records.
From www.contractsafe.com
Electronic Signature Examples and Types Sign a Contract in Seconds Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In march of 1997, fda issued final part 11 regulations that provide criteria for. Electronic Signature Policy For Medical Records.
From templates.rjuuc.edu.np
E Signature Policy Template Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. If. Electronic Signature Policy For Medical Records.
From machemapache.blogspot.com
Document Signature Format Free Documents Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In march of 1997, fda issued final part 11 regulations that provide criteria for. Electronic Signature Policy For Medical Records.
From www.eleapsoftware.com
21 CFR Part 11 Electronic Signature Requirements and Your LMS Electronic Signature Policy For Medical Records If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. Electronic signatures — scope and application. We encourage you to send a complete medical record with proper signature documentation first. Electronic Signature Policy For Medical Records.
From www.scribd.com
Electronic Signatures Medical Records Authentication Medical Record Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. Electronic signatures — scope and application. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. In march of 1997, fda issued final. Electronic Signature Policy For Medical Records.
From www.esigngenie.com
Doctors Signature Easily Create and Manage eSignatures Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. In march of 1997, fda issued final part 11 regulations that provide criteria for. Electronic Signature Policy For Medical Records.
From old.sermitsiaq.ag
E Signature Policy Template Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information. Electronic Signature Policy For Medical Records.
From blog.pdfliner.com
Doctor Signature How to Add Electronic Physician Signatures Digitally Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of. Electronic Signature Policy For Medical Records.
From www.scribd.com
FDA DRAFT Guidance Use of Electronic Records and Electronic Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. Electronic signatures — scope and application. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment. Electronic Signature Policy For Medical Records.
From templates.rjuuc.edu.np
E Signature Policy Template Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. In december 2022, the centers for medicare and medicaid services (cms) published a. Electronic Signature Policy For Medical Records.
From www.fillhq.com
Electronic Signature for Healthcare Fill Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In march of 1997, fda issued final part 11 regulations that provide criteria. Electronic Signature Policy For Medical Records.
From workhub.ai
How Can Healthcare Be Helped by Electronic Signature Software? Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. Electronic signatures — scope and application. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. We encourage you to send a complete medical record. Electronic Signature Policy For Medical Records.
From www.slideserve.com
PPT Electronic Health Record (EHR) Signatures PowerPoint Presentation Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. Electronic signatures — scope and application. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. If the handwritten signature is illegible, include a signature log (if electronic, include the. Electronic Signature Policy For Medical Records.
From printabletemplate.conaresvirtual.edu.sv
E Signature Policy Template Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. If you practice telehealth, keep. Electronic Signature Policy For Medical Records.
From old.sermitsiaq.ag
E Signature Policy Template Electronic Signature Policy For Medical Records Electronic signatures — scope and application. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. We encourage you to send. Electronic Signature Policy For Medical Records.
From old.sermitsiaq.ag
Signature Authorization Form Template Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol). Electronic Signature Policy For Medical Records.
From caredirectives.zendesk.com
Electronic Patient and Physician Signatures Care Directives Electronic Signature Policy For Medical Records If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. Electronic signatures — scope and application. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If the handwritten signature is illegible, include a signature log (if electronic, include the. Electronic Signature Policy For Medical Records.
From www.signnow.com
Electronic Signature Complete with ease airSlate SignNow Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If the handwritten signature is illegible, include a signature log (if. Electronic Signature Policy For Medical Records.
From workhub.ai
Electronic Signature Examples An Overview Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. In march of 1997, fda. Electronic Signature Policy For Medical Records.
From www.pandadoc.com
Onboard an Electronic Signature Policy for Medical Records Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. Electronic signatures — scope and application. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In march of 1997, fda issued final part 11. Electronic Signature Policy For Medical Records.
From educationfun1.com
CFR Part 11 GMP Electronic Signatures Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. Electronic signatures — scope and application. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If the handwritten signature is illegible, include a. Electronic Signature Policy For Medical Records.
From old.sermitsiaq.ag
Electronic Medical Records Policy And Procedure Template Electronic Signature Policy For Medical Records Electronic signatures — scope and application. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. We encourage you to. Electronic Signature Policy For Medical Records.
From old.sermitsiaq.ag
E Signature Policy Template Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. Electronic signatures — scope and application. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. We encourage you to. Electronic Signature Policy For Medical Records.
From www.youtube.com
Digital Signature Algorithm Simple Example xRay Pixy YouTube Electronic Signature Policy For Medical Records In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. Electronic signatures — scope and application. In march of 1997, fda issued final part 11. Electronic Signature Policy For Medical Records.
From issuu.com
Implementation of Electronic Signatures in Healthcare Organizations Electronic Signature Policy For Medical Records If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance. Electronic Signature Policy For Medical Records.
From www.researchgate.net
(PDF) Legality for Electronic Signatures in Implementing Electronic Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. Electronic signatures — scope and application. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment. Electronic Signature Policy For Medical Records.
From www.scilife.io
21 CFR Part 11 Compliance Definition, Requirements and Implications Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. In march of 1997, fda issued final part 11 regulations that provide criteria for. Electronic Signature Policy For Medical Records.
From www.plus91online.com
Electronic Medical Record(EMR ),a traditional paper based medical Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In march of 1997, fda issued final part 11 regulations that provide criteria. Electronic Signature Policy For Medical Records.
From calystaemr.com
The Digital Doctor Will See You Now A Guide to Electronic Signatures Electronic Signature Policy For Medical Records In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information. Electronic Signature Policy For Medical Records.
From printabletemplate.conaresvirtual.edu.sv
Electronic Signature Policy Template Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. If you practice telehealth, keep electronic medical or health records. Electronic Signature Policy For Medical Records.
From www.esigngenie.com
HIPAA Compliant e Signature HIPAA Compliant Electronic Signature Electronic Signature Policy For Medical Records We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In december 2022, the centers for medicare and medicaid services (cms) published a. Electronic Signature Policy For Medical Records.
From www.simplerqms.com
21 CFR Part 11 Compliant Electronic Signatures (Essential Guide) Electronic Signature Policy For Medical Records If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. In december 2022, the centers for medicare and medicaid services (cms) published a proposed rule which has the objective of resolving an issue with healthcare attachment transactions. Electronic signatures — scope and application. We encourage you to send a complete medical record. Electronic Signature Policy For Medical Records.
From www.youtube.com
Electronic records and electronic signatures according to 21 CFR Part Electronic Signature Policy For Medical Records If the handwritten signature is illegible, include a signature log (if electronic, include the protocol) provider compliance has more information about how to avoid common coverage, coding, and. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda, under certain circumstances, of electronic. In december 2022, the centers for medicare and medicaid services. Electronic Signature Policy For Medical Records.
From www.zoho.com
FDA 21 CFR Part 11 compliance with electronic signatures Zoho Sign Electronic Signature Policy For Medical Records Electronic signatures — scope and application. If you practice telehealth, keep electronic medical or health records (emrs/ehrs), or conduct business with parties over a. We encourage you to send a complete medical record with proper signature documentation first to avoid medical review delays. In march of 1997, fda issued final part 11 regulations that provide criteria for acceptance by fda,. Electronic Signature Policy For Medical Records.