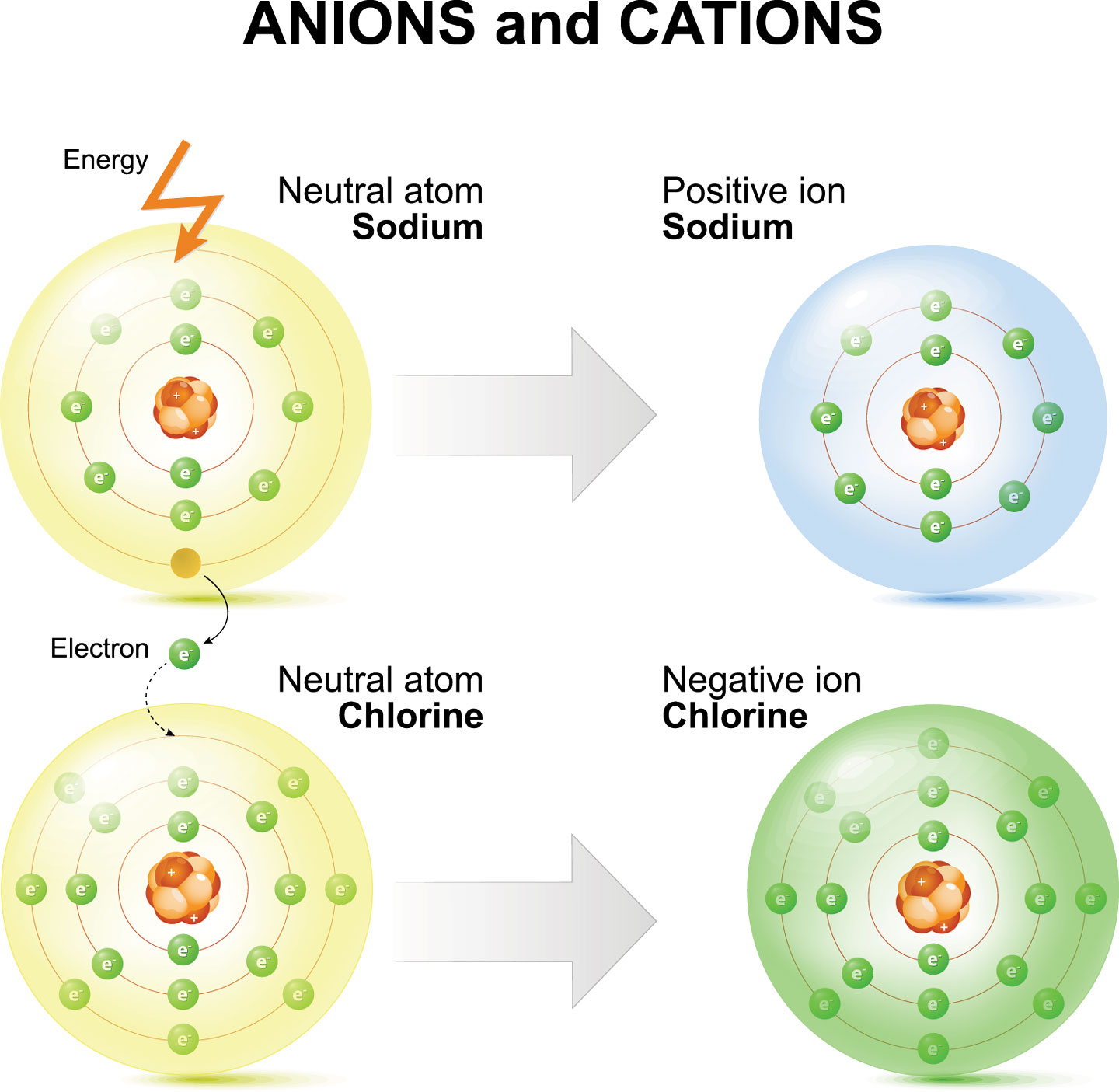
Chloride Ion Larger Than Atom . Is a chlorine atom larger than a chloride ion? An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. The proton number of both chlorine and chloride ion does not change, but remains at 17. Anions are therefore larger than the parent atom for ions of the same charge (e.g. The van der waals radius is the distance. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Hence, we can deduce that the forces of.
from exofbwxrr.blob.core.windows.net
The van der waals radius is the distance. Anions are therefore larger than the parent atom for ions of the same charge (e.g. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. Hence, we can deduce that the forces of. The proton number of both chlorine and chloride ion does not change, but remains at 17. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Is a chlorine atom larger than a chloride ion?
Chloride Ion Of Protons at James Kirts blog
Chloride Ion Larger Than Atom The proton number of both chlorine and chloride ion does not change, but remains at 17. The van der waals radius is the distance. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Anions are therefore larger than the parent atom for ions of the same charge (e.g. Is a chlorine atom larger than a chloride ion? In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The proton number of both chlorine and chloride ion does not change, but remains at 17. Hence, we can deduce that the forces of. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chloride Ion Larger Than Atom An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The van der waals radius is the distance. In the same group) the size increases as we go down a group in the periodic table. Chloride Ion Larger Than Atom.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chloride Ion Larger Than Atom The proton number of both chlorine and chloride ion does not change, but remains at 17. Is a chlorine atom larger than a chloride ion? Hence, we can deduce that the forces of. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the. Chloride Ion Larger Than Atom.
From sciencenotes.org
Atomic Radius and Ionic Radius Chloride Ion Larger Than Atom Is a chlorine atom larger than a chloride ion? An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The van der waals radius is the distance. Hence, we can deduce that the forces of.. Chloride Ion Larger Than Atom.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID1073512 Chloride Ion Larger Than Atom Is a chlorine atom larger than a chloride ion? In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The van der waals radius is the distance. Anions are therefore larger than the parent atom for. Chloride Ion Larger Than Atom.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chloride Ion Larger Than Atom The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. In the same group) the size. Chloride Ion Larger Than Atom.
From slideplayer.com
Periodic Table & Periodic Trends ppt download Chloride Ion Larger Than Atom The van der waals radius is the distance. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Hence, we can deduce that the forces of. Is a chlorine atom larger than a chloride ion? The. Chloride Ion Larger Than Atom.
From ian-has-logan.blogspot.com
How Do Chemists Model the Valence Electrons in Metal Atoms IanhasLogan Chloride Ion Larger Than Atom Anions are therefore larger than the parent atom for ions of the same charge (e.g. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. The van der waals radius is the distance. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a. Chloride Ion Larger Than Atom.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Larry Alford blog Chloride Ion Larger Than Atom The proton number of both chlorine and chloride ion does not change, but remains at 17. Hence, we can deduce that the forces of. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The covalent. Chloride Ion Larger Than Atom.
From www.chegg.com
Solved 15) Why is the ionic radius of a chloride ion larger Chloride Ion Larger Than Atom The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. The van der waals radius is the distance. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl. Chloride Ion Larger Than Atom.
From www.savemyexams.co.uk
Ions & Ionic Bonds (2.2.1) CIE IGCSE Chemistry Revision Notes 2023 Save My Exams Chloride Ion Larger Than Atom Anions are therefore larger than the parent atom for ions of the same charge (e.g. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Hence, we can deduce that the forces of. An atom such. Chloride Ion Larger Than Atom.
From chem.libretexts.org
7.3 Sizes of Atoms and Ions Chemistry LibreTexts Chloride Ion Larger Than Atom An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. The proton number of both chlorine. Chloride Ion Larger Than Atom.
From exofbwxrr.blob.core.windows.net
Chloride Ion Of Protons at James Kirts blog Chloride Ion Larger Than Atom An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The proton number of both chlorine and chloride ion does not change, but remains at 17. The van der waals radius is the distance. The. Chloride Ion Larger Than Atom.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chloride Ion Larger Than Atom The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. In the same group) the size. Chloride Ion Larger Than Atom.
From www.numerade.com
SOLVED Sodium atoms are much larger than chlorine atoms, but sodium ions are much smaller than Chloride Ion Larger Than Atom The van der waals radius is the distance. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Anions are therefore larger than the parent atom for ions of the same charge (e.g. The covalent. Chloride Ion Larger Than Atom.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chloride Ion Larger Than Atom The proton number of both chlorine and chloride ion does not change, but remains at 17. Hence, we can deduce that the forces of. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Anions. Chloride Ion Larger Than Atom.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − ) when it gains an Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The proton number of both chlorine and chloride ion does not change, but remains at 17. Is a chlorine atom larger than a chloride ion? Hence,. Chloride Ion Larger Than Atom.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chloride Ion Larger Than Atom The van der waals radius is the distance. The proton number of both chlorine and chloride ion does not change, but remains at 17. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Hence,. Chloride Ion Larger Than Atom.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chloride Ion Larger Than Atom The van der waals radius is the distance. Anions are therefore larger than the parent atom for ions of the same charge (e.g. Hence, we can deduce that the forces of. Is a chlorine atom larger than a chloride ion? An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl. Chloride Ion Larger Than Atom.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chloride Ion Larger Than Atom Anions are therefore larger than the parent atom for ions of the same charge (e.g. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The van der waals radius is the distance. The proton number. Chloride Ion Larger Than Atom.
From www.chem.fsu.edu
Electron Configurations Chloride Ion Larger Than Atom The van der waals radius is the distance. Is a chlorine atom larger than a chloride ion? Hence, we can deduce that the forces of. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in.. Chloride Ion Larger Than Atom.
From www.youtube.com
Chlorine Electron Configuration YouTube Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Is a chlorine atom larger than a chloride ion? The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule. Chloride Ion Larger Than Atom.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom Chlorine atom Sodium ion Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The proton number of both chlorine and chloride ion does not change, but remains at 17. Is a chlorine atom larger than a chloride ion? Anions. Chloride Ion Larger Than Atom.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chloride Ion Larger Than Atom An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Hence, we can deduce that the forces of. The proton number of both chlorine and chloride ion does not change, but remains at 17. Anions. Chloride Ion Larger Than Atom.
From differencebtw.com
Chlorine Atom vs. Chloride Ion Know the Difference Chloride Ion Larger Than Atom The van der waals radius is the distance. The proton number of both chlorine and chloride ion does not change, but remains at 17. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase An atom. Chloride Ion Larger Than Atom.
From www.mooramo.com
The Formation of Ionic Compounds From Atoms Mooramo Chloride Ion Larger Than Atom Is a chlorine atom larger than a chloride ion? The proton number of both chlorine and chloride ion does not change, but remains at 17. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in.. Chloride Ion Larger Than Atom.
From www.science-revision.co.uk
Atoms to ions Chloride Ion Larger Than Atom The van der waals radius is the distance. Hence, we can deduce that the forces of. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase An atom such as chlorine has both a covalent radius. Chloride Ion Larger Than Atom.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine atom chemical reaction Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Hence, we can deduce that the forces of. Anions are therefore larger than the parent atom for ions of the same charge (e.g. The covalent radius. Chloride Ion Larger Than Atom.
From chem.libretexts.org
3.1 Ionic Atoms Chemistry LibreTexts Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase The proton number of both chlorine and chloride ion does not change, but remains at 17. The covalent radius of cl2 is the distance between the. Chloride Ion Larger Than Atom.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chloride Ion Larger Than Atom An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. Anions are therefore larger than the parent atom for ions of the same charge (e.g. In the same group) the size increases as we go. Chloride Ion Larger Than Atom.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding PowerPoint Presentation, free download ID398203 Chloride Ion Larger Than Atom Is a chlorine atom larger than a chloride ion? Anions are therefore larger than the parent atom for ions of the same charge (e.g. The covalent radius of cl2 is the distance between the two chlorine atoms in a single molecule of cl2. An atom such as chlorine has both a covalent radius (the distance between the two atoms in. Chloride Ion Larger Than Atom.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Chloride Ion Larger Than Atom Anions are therefore larger than the parent atom for ions of the same charge (e.g. Hence, we can deduce that the forces of. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The proton. Chloride Ion Larger Than Atom.
From slideplayer.com
Elemental Properties and Patterns ppt download Chloride Ion Larger Than Atom The van der waals radius is the distance. Is a chlorine atom larger than a chloride ion? Hence, we can deduce that the forces of. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Anions. Chloride Ion Larger Than Atom.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion Larger Than Atom Hence, we can deduce that the forces of. The van der waals radius is the distance. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in. The covalent radius of cl2 is the distance between. Chloride Ion Larger Than Atom.
From gardenandplate.com
Molecules Chloride Ion Larger Than Atom In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom and the ion will increase Hence, we can deduce that the forces of. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a. Chloride Ion Larger Than Atom.
From slideplayer.com
Chemical Bonds The Formation of Compounds From Atoms ppt download Chloride Ion Larger Than Atom The proton number of both chlorine and chloride ion does not change, but remains at 17. Anions are therefore larger than the parent atom for ions of the same charge (e.g. In the same group) the size increases as we go down a group in the periodic table as the principle quantum increases the size of both the parent atom. Chloride Ion Larger Than Atom.