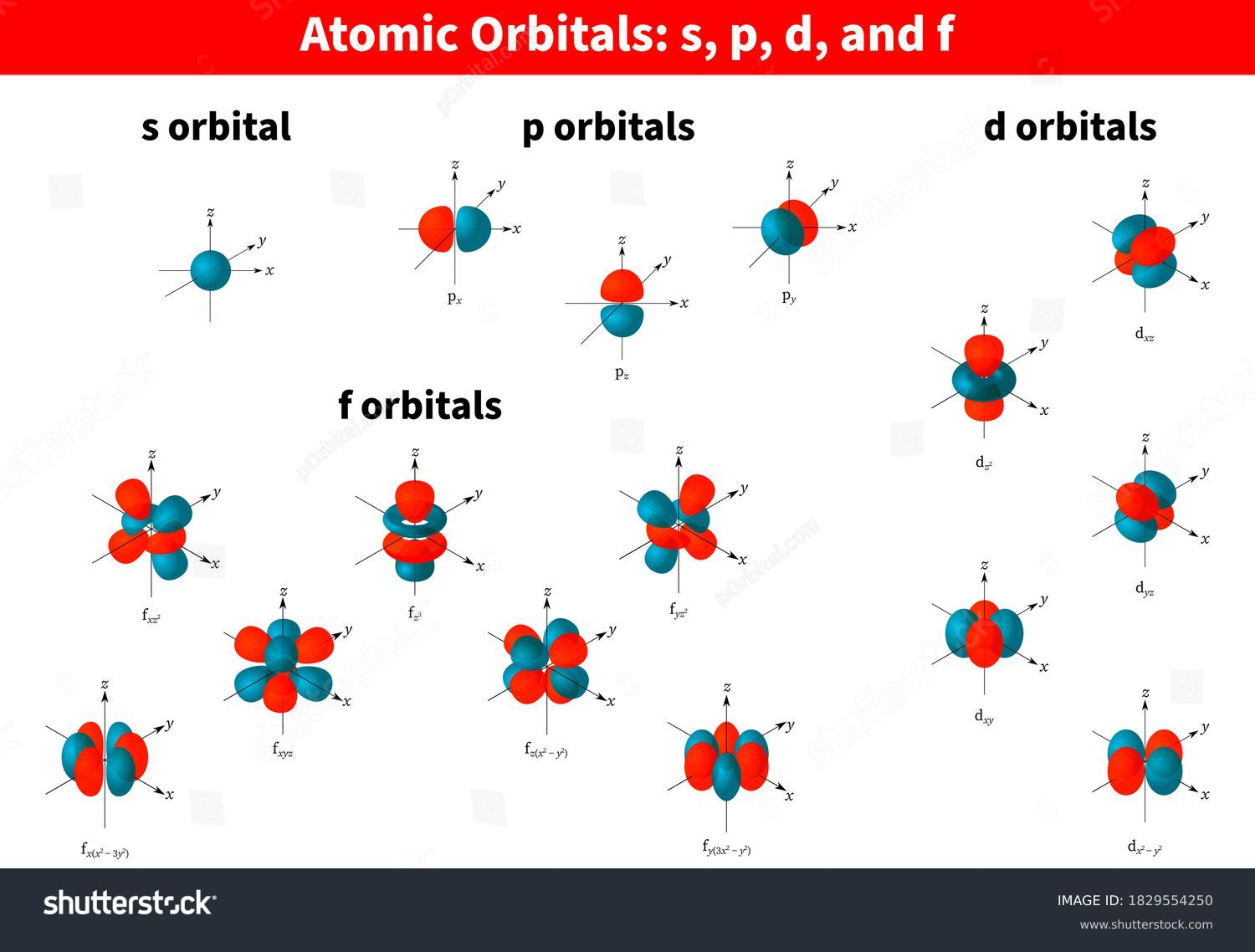
What Is The Meaning Of S P D F In Electron Configuration . The maximum number of electrons that can be accommodated by a subshell. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The letter refers to the shape of the orbital. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f. The letters go in the order s, p, d, f, g, h, i, j, etc. Each successive integer typically represents a higher energy level than the last. These subshells are called as s, p, d, or f. Letters like s, p, d, and f are the subshells. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin.
from www.shutterstock.com
These subshells are called as s, p, d, or f. Letters like s, p, d, and f are the subshells. The letter refers to the shape of the orbital. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. Each successive integer typically represents a higher energy level than the last. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The letters go in the order s, p, d, f, g, h, i, j, etc. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f.
Atomic Orbitals S P D F 库存插图 1829554250 Shutterstock
What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. These subshells are called as s, p, d, or f. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The p, d, and f. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. Each successive integer typically represents a higher energy level than the last. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. Letters like s, p, d, and f are the subshells. The letter refers to the shape of the orbital. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The maximum number of electrons that can be accommodated by a subshell. The letters go in the order s, p, d, f, g, h, i, j, etc.
From www.youtube.com
Electron Configuration Basic introduction YouTube What Is The Meaning Of S P D F In Electron Configuration The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The p, d, and f. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The four different types of orbitals (s,p,d,. What Is The Meaning Of S P D F In Electron Configuration.
From pages.swcp.com
Parsing the spdf electron orbital model What Is The Meaning Of S P D F In Electron Configuration Letters like s, p, d, and f are the subshells. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The letter refers to the shape of the orbital. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the. What Is The Meaning Of S P D F In Electron Configuration.
From www.youtube.com
Electron configuration spdf notation Part 2 YouTube What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f. The letters s, p, d, and f were assigned for historical reasons that need not concern us. Letters like s, p,. What Is The Meaning Of S P D F In Electron Configuration.
From chemistry291.blogspot.com
[] What Is the Fluorine(F) Electron Configuration? What Is The Meaning Of S P D F In Electron Configuration The letters s, p, d, and f were assigned for historical reasons that need not concern us. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The letter refers to the shape of the orbital. The maximum number of electrons that. What Is The Meaning Of S P D F In Electron Configuration.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki What Is The Meaning Of S P D F In Electron Configuration The maximum number of electrons that can be accommodated by a subshell. Letters like s, p, d, and f are the subshells. The letter refers to the shape of the orbital. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The. What Is The Meaning Of S P D F In Electron Configuration.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively.. What Is The Meaning Of S P D F In Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The maximum number of electrons that can be accommodated by a subshell. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. Letters like s, p, d, and f are the subshells. The letters s, p, d, and. What Is The Meaning Of S P D F In Electron Configuration.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is The Meaning Of S P D F In Electron Configuration The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The letter refers to the shape of the orbital. Letters. What Is The Meaning Of S P D F In Electron Configuration.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The p, d, and f. The letter refers to the shape of the orbital. The maximum number of electrons that can be accommodated by a subshell. These subshells are called as s, p, d, or f. Letters like s, p, d, and f are the subshells. The letters. What Is The Meaning Of S P D F In Electron Configuration.
From www.youtube.com
Electron Configuration What is it? What are S P D F subshells? How to write configurations What Is The Meaning Of S P D F In Electron Configuration The letter refers to the shape of the orbital. Letters like s, p, d, and f are the subshells. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two. What Is The Meaning Of S P D F In Electron Configuration.
From www.youtube.com
Electron Configurations SPDF and Kernel Notation Notes YouTube What Is The Meaning Of S P D F In Electron Configuration The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. Letters like s, p, d, and f are the subshells. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The letter. What Is The Meaning Of S P D F In Electron Configuration.
From socratic.org
s,p,d,f Orbitals Chemistry Socratic What Is The Meaning Of S P D F In Electron Configuration The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The. What Is The Meaning Of S P D F In Electron Configuration.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Meaning Of S P D F In Electron Configuration Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The maximum number of electrons that can be accommodated by a subshell. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively.. What Is The Meaning Of S P D F In Electron Configuration.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica What Is The Meaning Of S P D F In Electron Configuration The letter refers to the shape of the orbital. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The four different. What Is The Meaning Of S P D F In Electron Configuration.
From www.slideserve.com
PPT Pick Up Electron Notes Periodic Table 4 different colored pencils PowerPoint Presentation What Is The Meaning Of S P D F In Electron Configuration These subshells are called as s, p, d, or f. The maximum number of electrons that can be accommodated by a subshell. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron. What Is The Meaning Of S P D F In Electron Configuration.
From slidetodoc.com
Electron Configuration s p d and f The What Is The Meaning Of S P D F In Electron Configuration Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Each successive integer typically represents a higher energy level than the last. The letters go in the order. What Is The Meaning Of S P D F In Electron Configuration.
From www.breakingatom.com
Advanced Electron Configuration What Is The Meaning Of S P D F In Electron Configuration Letters like s, p, d, and f are the subshells. The p, d, and f. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The letter refers to the shape of the orbital. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with. What Is The Meaning Of S P D F In Electron Configuration.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is The Meaning Of S P D F In Electron Configuration The letters go in the order s, p, d, f, g, h, i, j, etc. Letters like s, p, d, and f are the subshells. These subshells are called as s, p, d, or f. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2,. What Is The Meaning Of S P D F In Electron Configuration.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry What Is The Meaning Of S P D F In Electron Configuration Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The letters go in the order s, p, d, f, g, h, i, j, etc. The p, d, and f. Each successive integer typically represents a higher energy level than the last. The letter refers to the shape of the. What Is The Meaning Of S P D F In Electron Configuration.
From byjus.com
What is the value of s,p,d.f? What Is The Meaning Of S P D F In Electron Configuration The p, d, and f. The letters go in the order s, p, d, f, g, h, i, j, etc. The maximum number of electrons that can be accommodated by a subshell. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The simple names s orbital, p orbital, d. What Is The Meaning Of S P D F In Electron Configuration.
From socratic.org
Electron Configuration Chemistry Socratic What Is The Meaning Of S P D F In Electron Configuration The letter refers to the shape of the orbital. The letters go in the order s, p, d, f, g, h, i, j, etc. The p, d, and f. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The simple names s orbital, p orbital, d orbital,. What Is The Meaning Of S P D F In Electron Configuration.
From www.geeksforgeeks.org
Shapes of Atomic Orbitals Shape of s, p, d, f Orbitals, FAQs, Examples What Is The Meaning Of S P D F In Electron Configuration The p, d, and f. Each successive integer typically represents a higher energy level than the last. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and. What Is The Meaning Of S P D F In Electron Configuration.
From www.youtube.com
Write the general outer electronic configuration of `s`,`p`,`d` and `fblock` elements. YouTube What Is The Meaning Of S P D F In Electron Configuration The letter refers to the shape of the orbital. These subshells are called as s, p, d, or f. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The letters go in the order s, p, d, f, g, h, i, j, etc. Each successive integer typically represents a higher energy level. What Is The Meaning Of S P D F In Electron Configuration.
From www.thoughtco.com
Electron Configuration Chart What Is The Meaning Of S P D F In Electron Configuration The maximum number of electrons that can be accommodated by a subshell. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The letters go in the order s, p, d, f, g, h, i, j, etc. The simple names s orbital, p orbital, d orbital, and f orbital refer. What Is The Meaning Of S P D F In Electron Configuration.
From www.pinterest.com.mx
The spdf orbitals Chemistry education, Teaching chemistry, Chemistry classroom What Is The Meaning Of S P D F In Electron Configuration The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Each successive integer typically represents a higher energy level than the last. The p, d, and f. These subshells are called as s, p, d, or f. Letters like s, p, d, and f are the subshells. The. What Is The Meaning Of S P D F In Electron Configuration.
From www.shutterstock.com
Atomic Orbitals S P D F 库存插图 1829554250 Shutterstock What Is The Meaning Of S P D F In Electron Configuration Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The letter refers to the shape of the orbital. The p, d, and f. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and. What Is The Meaning Of S P D F In Electron Configuration.
From chemistry.stackexchange.com
electrons What is SPDF configuration? Chemistry Stack Exchange What Is The Meaning Of S P D F In Electron Configuration The letters go in the order s, p, d, f, g, h, i, j, etc. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. Letters like s, p, d, and f are the subshells. These subshells are called as s, p, d, or f. The four different types of. What Is The Meaning Of S P D F In Electron Configuration.
From manualworshipped.z14.web.core.windows.net
Orbital Diagram S P D F What Is The Meaning Of S P D F In Electron Configuration The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The letter refers to the shape of the orbital. These subshells are called as s, p, d, or f. The. What Is The Meaning Of S P D F In Electron Configuration.
From abacus.bates.edu
Chem Notes What Is The Meaning Of S P D F In Electron Configuration The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. These subshells are called as s, p, d, or f. The letter. What Is The Meaning Of S P D F In Electron Configuration.
From www.youtube.com
F Electron Configuration (Fluoride Ion) YouTube What Is The Meaning Of S P D F In Electron Configuration The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The p, d, and f. Based on hund's rule, one electron fills each p \rm p p orbital, and each electron has the same spin. The subshells correspond to l=0, l=1, l=2,. What Is The Meaning Of S P D F In Electron Configuration.
From www.showme.com
S. P. D. F. Electron blocks on the periodic table Chemistry, Periodic Table, Electron What Is The Meaning Of S P D F In Electron Configuration The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Each successive integer typically represents a higher energy level than the last. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The maximum number of electrons that can be accommodated. What Is The Meaning Of S P D F In Electron Configuration.
From www.animalia-life.club
Electron Configuration What Is The Meaning Of S P D F In Electron Configuration The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. The letters go in the order s, p, d, f, g, h, i, j, etc. The maximum number of electrons that can be accommodated by a subshell. Based on hund's rule, one. What Is The Meaning Of S P D F In Electron Configuration.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. These subshells are called as s, p, d, or f. The letters s, p, d, and f were assigned for historical reasons that need not concern us. The. What Is The Meaning Of S P D F In Electron Configuration.
From chem.libretexts.org
7.8B Electron Configurations and the Periodic Table Chemistry LibreTexts What Is The Meaning Of S P D F In Electron Configuration The letters go in the order s, p, d, f, g, h, i, j, etc. Letters like s, p, d, and f are the subshells. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. Each successive integer typically represents a higher. What Is The Meaning Of S P D F In Electron Configuration.
From www.pinterest.com
Electron Configurations A Must Know Hack in 2021 Electron configuration, Electrons, Chemistry What Is The Meaning Of S P D F In Electron Configuration Each successive integer typically represents a higher energy level than the last. The p, d, and f. The letter refers to the shape of the orbital. The letters go in the order s, p, d, f, g, h, i, j, etc. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum. What Is The Meaning Of S P D F In Electron Configuration.