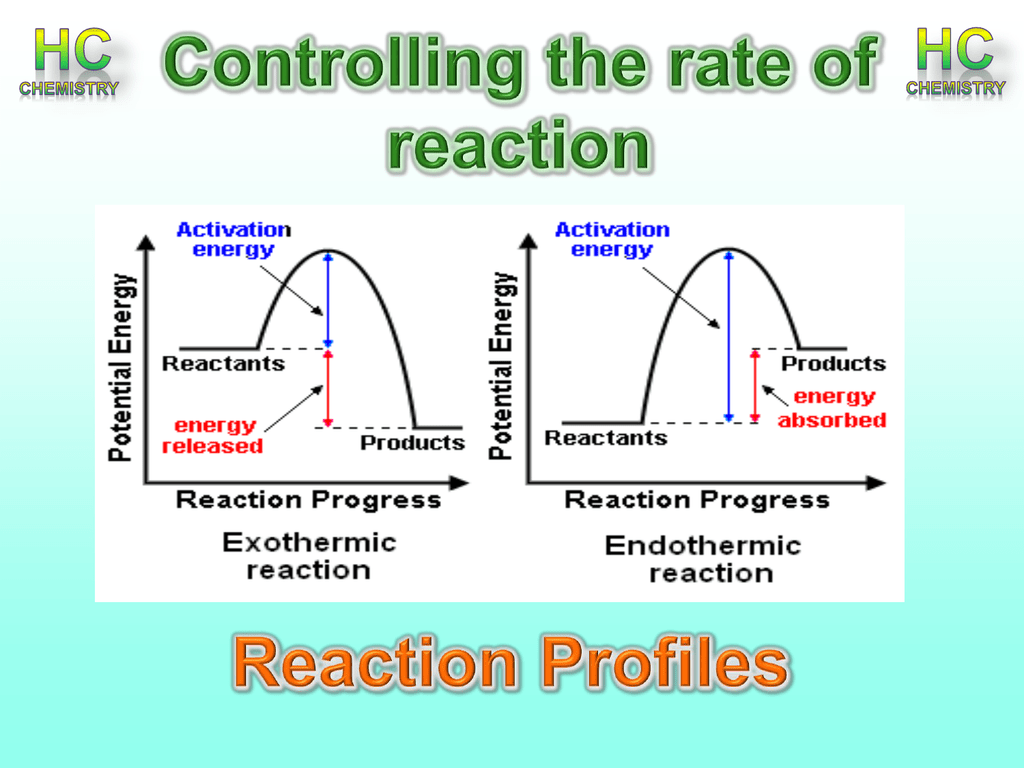
Endothermic Reaction Bbc . The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Sports injury treatment often use cold packs based on. If more energy is absorbed than is released, this reaction is endothermic. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some examples of endothermic reactions are: Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. More energy is required to break the bonds than is released from making the. The reaction between ethanoic acid and sodium carbonate. These reactions can also be very useful, such as.
from mavink.com
Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some examples of endothermic reactions are: Sports injury treatment often use cold packs based on. If more energy is absorbed than is released, this reaction is endothermic. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. The reaction between ethanoic acid and sodium carbonate. More energy is required to break the bonds than is released from making the. These reactions can also be very useful, such as. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their.
Endothermic Reaction Profile Diagram
Endothermic Reaction Bbc More energy is required to break the bonds than is released from making the. More energy is required to break the bonds than is released from making the. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Sports injury treatment often use cold packs based on. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Some examples of endothermic reactions are: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The reaction between ethanoic acid and sodium carbonate. If more energy is absorbed than is released, this reaction is endothermic. These reactions can also be very useful, such as.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Some examples of endothermic reactions are: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. More energy is. Endothermic Reaction Bbc.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Bbc Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. These reactions can also be very useful, such as. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. An exothermic chemical reaction causes an increase in temperature (of the surroundings). If more energy is absorbed than is released, this. Endothermic Reaction Bbc.
From www.vrogue.co
Igcse Gcse Chemistry Exothermic And Endothermic Reactions Exercise Vrogue Endothermic Reaction Bbc Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Sports injury treatment often use cold packs based on. Some examples of endothermic reactions are: Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. The reaction between ethanoic acid and sodium carbonate. More energy is required. Endothermic Reaction Bbc.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. More energy is required to break the bonds than is released from making the. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Sports injury treatment often use cold packs based on. These reactions can also be. Endothermic Reaction Bbc.
From www.bbc.co.uk
Exothermic and endothermic reactions AQA test questions GCSE Endothermic Reaction Bbc In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The reaction between ethanoic acid and sodium carbonate. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). More energy is required to break the bonds than. Endothermic Reaction Bbc.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Bbc Sports injury treatment often use cold packs based on. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. These reactions can also be very useful, such as. Endothermic reactions absorb energy, resulting in a decrease in the surrounding. Endothermic Reaction Bbc.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Bbc Some examples of endothermic reactions are: An exothermic chemical reaction causes an increase in temperature (of the surroundings). The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. In an endothermic process, the heat that a system absorbs is thermal. Endothermic Reaction Bbc.
From www.bbc.co.uk
BBC Two Bitesize Science, Endothermic and exothermic reactions Endothermic Reaction Bbc Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. More energy is required to break the bonds than is released from making the. Some examples of endothermic reactions are: If more energy is absorbed than is released, this reaction is endothermic. Sports injury treatment often use cold packs based on. Revise and understand what endothermic. Endothermic Reaction Bbc.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The reaction between ethanoic acid and sodium carbonate. In. Endothermic Reaction Bbc.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Bbc Sports injury treatment often use cold packs based on. Some examples of endothermic reactions are: More energy is required to break the bonds than is released from making the. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The reaction between ethanoic acid and sodium carbonate. These reactions can also be very. Endothermic Reaction Bbc.
From www.coursehero.com
[Solved] Describe the difference between an exothermic and endothermic Endothermic Reaction Bbc Some examples of endothermic reactions are: If more energy is absorbed than is released, this reaction is endothermic. These reactions can also be very useful, such as. More energy is required to break the bonds than is released from making the. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Endothermic Reaction Bbc.
From www.pinterest.co.uk
BBC GCSE Bitesize Exothermic and endothermic reactions Science Endothermic Reaction Bbc Sports injury treatment often use cold packs based on. An exothermic chemical reaction causes an increase in temperature (of the surroundings). The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. These reactions can also be very useful, such. Endothermic Reaction Bbc.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Bbc Sports injury treatment often use cold packs based on. If more energy is absorbed than is released, this reaction is endothermic. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Revise and understand what endothermic and exothermic reactions are and how the two. Endothermic Reaction Bbc.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction Bbc Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. If more energy is absorbed than is released, this reaction is endothermic. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Some examples of endothermic reactions are: In an endothermic process,. Endothermic Reaction Bbc.
From www.bbc.co.uk
Chemistry KS3/GCSE Endothermic and exothermic reactions BBC Teach Endothermic Reaction Bbc Sports injury treatment often use cold packs based on. The reaction between ethanoic acid and sodium carbonate. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. If more energy is absorbed than is released, this reaction is endothermic. More energy is required to break the bonds than is released from making. Endothermic Reaction Bbc.
From www.bbc.co.uk
Chemistry KS3/GCSE Endothermic and exothermic reactions BBC Teach Endothermic Reaction Bbc More energy is required to break the bonds than is released from making the. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Sports injury treatment often use cold packs based on. If more energy is absorbed. Endothermic Reaction Bbc.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Some examples of endothermic reactions are: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An exothermic chemical reaction causes an increase in temperature (of the surroundings). If more energy is absorbed than is released, this reaction is. Endothermic Reaction Bbc.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc Some examples of endothermic reactions are: These reactions can also be very useful, such as. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. The reaction between ethanoic acid and. Endothermic Reaction Bbc.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Bbc In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. More energy is required to break the bonds than is released from making the. Some examples of endothermic reactions are: Sports injury treatment often use cold packs based on. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and. Endothermic Reaction Bbc.
From www.bbc.co.uk
Chemistry KS3/GCSE Endothermic and exothermic reactions BBC Teach Endothermic Reaction Bbc An exothermic chemical reaction causes an increase in temperature (of the surroundings). If more energy is absorbed than is released, this reaction is endothermic. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Sports injury treatment often use cold packs based on. Revise and understand what endothermic and exothermic reactions are and. Endothermic Reaction Bbc.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Endothermic Reaction Bbc These reactions can also be very useful, such as. The reaction between ethanoic acid and sodium carbonate. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Sports injury treatment often use cold packs based on. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. More energy is required to break the bonds. Endothermic Reaction Bbc.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction Bbc Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). More energy is required to break the bonds than is released from making the. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The reaction between. Endothermic Reaction Bbc.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Bbc Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. If more energy is absorbed than is released,. Endothermic Reaction Bbc.
From www.vrogue.co
Gcse Bbc Bitesize Higher Science Exothermic And Endot vrogue.co Endothermic Reaction Bbc If more energy is absorbed than is released, this reaction is endothermic. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. More energy is required to break the bonds than is released from making the. Endothermic reactions absorb energy, resulting in a decrease. Endothermic Reaction Bbc.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Bbc The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Some examples of endothermic reactions are: Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The reaction between ethanoic acid and sodium carbonate. Endothermic reactions absorb energy, resulting in a. Endothermic Reaction Bbc.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Bbc Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. If more energy is absorbed than is released, this reaction is endothermic. In an endothermic process, the heat that a system. Endothermic Reaction Bbc.
From diseno.librogratis.info
Britain For Learners 2nd Ed Reading Libro Gratis Endothermic Reaction Bbc If more energy is absorbed than is released, this reaction is endothermic. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Sports injury treatment often use cold packs based on. These reactions can also be very useful, such as.. Endothermic Reaction Bbc.
From diagramlibraryguanine.z19.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Reaction Bbc The reaction between ethanoic acid and sodium carbonate. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. These reactions can also be very useful, such as. If more energy is absorbed than is released, this reaction is endothermic. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system.. Endothermic Reaction Bbc.
From www.memory.com
Question Endothermic reaction Memory Endothermic Reaction Bbc More energy is required to break the bonds than is released from making the. The reaction between ethanoic acid and sodium carbonate. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. Some examples of endothermic reactions are: An exothermic chemical reaction causes an increase in temperature (of the surroundings). The slideshow describes an exothermic reaction between dilute. Endothermic Reaction Bbc.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Reaction Bbc Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. The reaction between ethanoic acid and sodium carbonate. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. More energy is required to break the bonds than is released from making the. The slideshow describes an exothermic. Endothermic Reaction Bbc.
From mavink.com
Endothermic Reaction Profile Diagram Endothermic Reaction Bbc In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. Some examples of endothermic reactions are: More energy. Endothermic Reaction Bbc.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Bbc The reaction between ethanoic acid and sodium carbonate. The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid, and an endothermic reaction between. More energy is required to break the bonds than is released from making the. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. If more energy is absorbed than. Endothermic Reaction Bbc.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Bbc Some examples of endothermic reactions are: More energy is required to break the bonds than is released from making the. The reaction between ethanoic acid and sodium carbonate. Sports injury treatment often use cold packs based on. Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions. In an endothermic process, the heat that a system. Endothermic Reaction Bbc.
From mungfali.com
Endothermic Reaction Diagram Endothermic Reaction Bbc These reactions can also be very useful, such as. More energy is required to break the bonds than is released from making the. The reaction between ethanoic acid and sodium carbonate. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system.. Endothermic Reaction Bbc.
From ar.inspiredpencil.com
Endothermic Reaction Examples Everyday Life Endothermic Reaction Bbc If more energy is absorbed than is released, this reaction is endothermic. More energy is required to break the bonds than is released from making the. These reactions can also be very useful, such as. Endothermic reactions absorb energy, resulting in a decrease in the surrounding temperature. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In. Endothermic Reaction Bbc.