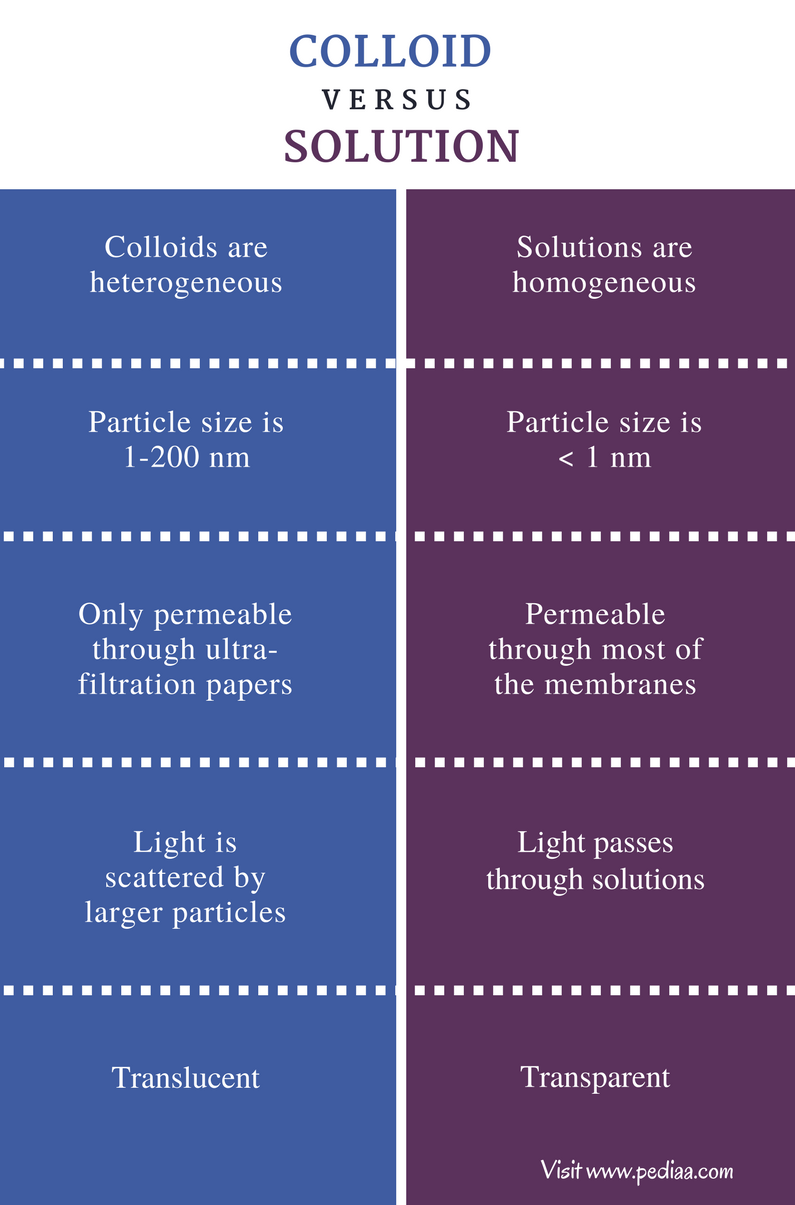
Colloid To Solution . Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Unlike true solutions where solute particles are dissolved at. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A beam of light passing through a true solution, such as air, is not visible. These particles can be solid, liquid, or gas. In a sense, they bridge the. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids can be distinguished from solutions using the tyndall effect. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. Light passing through a colloidal.
from pediaa.com
In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids can be distinguished from solutions using the tyndall effect. Unlike true solutions where solute particles are dissolved at. Light passing through a colloidal. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. A beam of light passing through a true solution, such as air, is not visible.
Difference Between Colloid and Solution Definition, Properties
Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A beam of light passing through a true solution, such as air, is not visible. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. These particles can be solid, liquid, or gas. Unlike true solutions where solute particles are dissolved at. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. Colloids can be distinguished from solutions using the tyndall effect. In a sense, they bridge the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Light passing through a colloidal.
From www.youtube.com
Types of Mixtures Solution, Suspension, Colloid NOTES YouTube Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A beam of light passing through a true solution, such as air, is not visible. Colloids can be distinguished from solutions using the tyndall effect. A solution may be colored, but it is transparent, the molecules or ions are invisible,. Colloid To Solution.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Colloid To Solution A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. In a sense, they bridge the. Unlike true solutions where solute particles are dissolved at. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In this chapter, we will consider the nature of solutions,. Colloid To Solution.
From studylib.net
Solutions, Colloids, Suspension Colloid To Solution In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloidal solution typically consists of particles ranging in size from 1 nanometer. Colloid To Solution.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Colloid To Solution These particles can be solid, liquid, or gas. A beam of light passing through a true solution, such as air, is not visible. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Colloids can be distinguished from solutions using the tyndall effect. A solution may. Colloid To Solution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Colloid To Solution A beam of light passing through a true solution, such as air, is not visible. Colloids can be distinguished from solutions using the tyndall effect. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties.. Colloid To Solution.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Colloid To Solution In a sense, they bridge the. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloidal solution typically consists of particles ranging in size from. Colloid To Solution.
From www.slideshare.net
Solutions & colloids Colloid To Solution A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Light passing through a colloidal. Unlike true solutions where solute particles are dissolved. Colloid To Solution.
From www.slideserve.com
PPT Fluid Management and Shock Resuscitation PowerPoint Presentation Colloid To Solution A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids can be distinguished from solutions using the tyndall effect. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A group of mixtures called colloids (or. Colloid To Solution.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Colloid To Solution Light passing through a colloidal. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. In a sense, they bridge the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to. Colloid To Solution.
From chemistrytalk.org
What Are Colloids? ChemTalk Colloid To Solution Unlike true solutions where solute particles are dissolved at. In a sense, they bridge the. Colloids can be distinguished from solutions using the tyndall effect. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloidal solution typically consists of particles ranging in size from 1. Colloid To Solution.
From pediaa.com
Difference Between True Solution and Colloidal Dispersion Definition Colloid To Solution A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Light passing through a colloidal. In a sense, they bridge the. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Colloids can. Colloid To Solution.
From collegedunia.com
Classification of Colloids Properties & Phases Colloid To Solution In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Colloids can be distinguished from solutions using the tyndall effect. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A group of mixtures called colloids. Colloid To Solution.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid To Solution These particles can be solid, liquid, or gas. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Light passing through a colloidal. In a sense, they bridge the. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution. Colloid To Solution.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Colloid To Solution A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Light passing through a colloidal. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Unlike true solutions where solute particles are dissolved at. A beam of. Colloid To Solution.
From www.pinterest.com
Colloid Easy Science Physics concepts, Organic chemistry study Colloid To Solution A beam of light passing through a true solution, such as air, is not visible. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. Colloids can be distinguished from solutions using the tyndall effect. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing.. Colloid To Solution.
From exopyyzzo.blob.core.windows.net
Is Vinegar A Solution Suspension Or Colloid at Valerie Blum blog Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they. Colloid To Solution.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid To Solution These particles can be solid, liquid, or gas. A beam of light passing through a true solution, such as air, is not visible. Unlike true solutions where solute particles are dissolved at. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. A group of mixtures. Colloid To Solution.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. A beam of light passing through a true solution, such as air, is not visible. Colloids can be distinguished from solutions using the tyndall effect. Light passing through. Colloid To Solution.
From www.askdifference.com
Colloid vs. Solution — What’s the Difference? Colloid To Solution These particles can be solid, liquid, or gas. In a sense, they bridge the. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing.. Colloid To Solution.
From www.vecteezy.com
Coagulation of Colloid Particles vector illustration 21669350 Vector Colloid To Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A beam of light passing through a true solution, such as air, is not visible. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In a sense, they bridge the.. Colloid To Solution.
From uen.pressbooks.pub
Colloids Introductory Chemistry Colloid To Solution In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Light passing through a colloidal. In a sense, they bridge the. Colloids can be distinguished from solutions using the tyndall effect. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. A solution may. Colloid To Solution.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid To Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In a sense, they bridge the. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids can be distinguished from solutions using the tyndall effect. A beam of light passing. Colloid To Solution.
From www.youtube.com
surfaceChemistry True solution, colloidal solution and suspension Colloid To Solution In a sense, they bridge the. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Colloids can be distinguished from solutions using the tyndall effect. These. Colloid To Solution.
From www.pinterest.es
Solutions, Colloids, Suspensions Science lessons, Chemistry lessons Colloid To Solution In a sense, they bridge the. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A solution may be colored, but it is transparent, the. Colloid To Solution.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloid To Solution Unlike true solutions where solute particles are dissolved at. In a sense, they bridge the. Colloids can be distinguished from solutions using the tyndall effect. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Light passing through a colloidal. A solution may be colored, but it is transparent, the molecules or ions are. Colloid To Solution.
From www.studypool.com
SOLUTION How will you distinguish a colloid from a solution Studypool Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A beam of light passing through a true solution, such as air, is not visible. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. In. Colloid To Solution.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Colloid To Solution Light passing through a colloidal. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. These particles can be solid, liquid, or gas. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloidal solution typically. Colloid To Solution.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Colloid To Solution Unlike true solutions where solute particles are dissolved at. A beam of light passing through a true solution, such as air, is not visible. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. These particles can be solid, liquid, or gas. Light passing through a colloidal. In this chapter, we will consider the nature of solutions, and. Colloid To Solution.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Colloid To Solution In a sense, they bridge the. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. A beam of light passing through a. Colloid To Solution.
From chem.libretexts.org
Colloids Chemistry LibreTexts Colloid To Solution A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and what properties it. In a sense, they bridge the. A colloidal solution typically consists of particles. Colloid To Solution.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Colloid To Solution In a sense, they bridge the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids can be distinguished from solutions using the tyndall effect. These particles can be solid, liquid, or gas. In this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form. Colloid To Solution.
From www.animalia-life.club
Example Of Colloid Mixture Colloid To Solution Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A beam of light passing through a true solution, such as air, is not visible. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In a sense, they bridge the. In this chapter,. Colloid To Solution.
From www.difference.wiki
Colloid vs. Solution What’s the Difference? Colloid To Solution Colloids can be distinguished from solutions using the tyndall effect. A beam of light passing through a true solution, such as air, is not visible. Unlike true solutions where solute particles are dissolved at. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Light passing through a colloidal. In a sense, they bridge. Colloid To Solution.
From www.brainkart.com
Classifications of Colloidal solution Surface Chemistry Colloid To Solution A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. Colloids can be distinguished from solutions using the tyndall effect. A beam of light passing through a true solution, such as air, is not visible. In a sense, they bridge the. These particles can be solid, liquid, or gas. A solution may be colored, but it is transparent,. Colloid To Solution.
From www.youtube.com
Science Quiz Solution, Suspension or Colloid ANY 10 YouTube Colloid To Solution A beam of light passing through a true solution, such as air, is not visible. These particles can be solid, liquid, or gas. A group of mixtures called colloids (or colloidal dispersions ) exhibit properties. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloidal. Colloid To Solution.