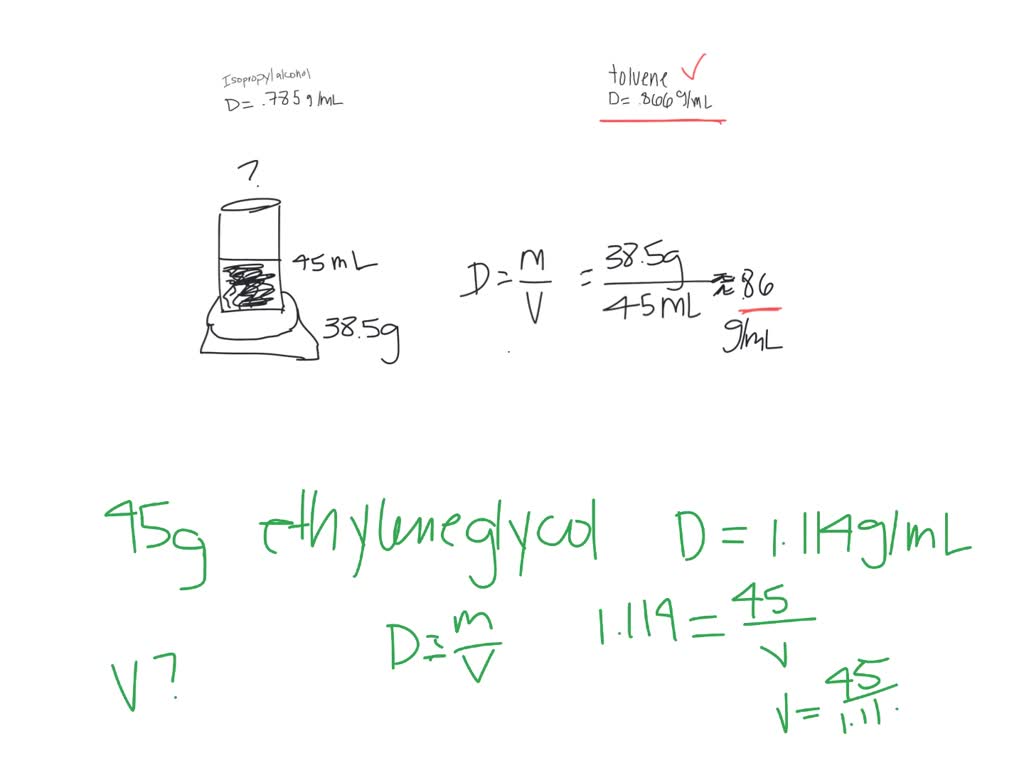
What Is The Density Of A Graduated Cylinder . A graduated cylinder contains 10.00 ml water. The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. Find mass using a balance scale, and use water displacement to find the volume. What is the density of. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). Suppose you place 3.31 ml of a substance into a graduated cylinder. By measuring the displacement of liquid when an object is. That means when you read the volume, you can estimate to the hundredths place. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. What is the density of the aluminum in g/ml? Use these values to determine the density of this. If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml).
from www.numerade.com
Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). Suppose you place 3.31 ml of a substance into a graduated cylinder. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. A graduated cylinder contains 10.00 ml water. Use these values to determine the density of this. By measuring the displacement of liquid when an object is. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. That means when you read the volume, you can estimate to the hundredths place. What is the density of.
SOLVED(a) To identify a liquid substance, a student determined its
What Is The Density Of A Graduated Cylinder A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. What is the density of. By measuring the displacement of liquid when an object is. If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. Suppose you place 3.31 ml of a substance into a graduated cylinder. Find mass using a balance scale, and use water displacement to find the volume. A graduated cylinder contains 10.00 ml water. The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. That means when you read the volume, you can estimate to the hundredths place. Use these values to determine the density of this. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. What is the density of the aluminum in g/ml?
From www.youtube.com
Measurement of density using a measuring cylinder YouTube What Is The Density Of A Graduated Cylinder What is the density of the aluminum in g/ml? Find mass using a balance scale, and use water displacement to find the volume. That means when you read the volume, you can estimate to the hundredths place. Use these values to determine the density of this. Suppose you place 3.31 ml of a substance into a graduated cylinder. A piece. What Is The Density Of A Graduated Cylinder.
From www.animalia-life.club
How To Read Graduated Cylinder Meniscus What Is The Density Of A Graduated Cylinder A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. What is the density of. That means when you read the volume, you can estimate to the hundredths place. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Calculating density. What Is The Density Of A Graduated Cylinder.
From courses.lumenlearning.com
Properties of Liquids Chemistry What Is The Density Of A Graduated Cylinder By measuring the displacement of liquid when an object is. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. If you look at a 10 ml graduated cylinder, for example, the. What Is The Density Of A Graduated Cylinder.
From www.pinterest.es
Pin on Chemistry Laboratory What Is The Density Of A Graduated Cylinder Suppose you place 3.31 ml of a substance into a graduated cylinder. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). Find mass using a balance scale, and use water displacement to find the volume. The graduated cylinder has a mass of 12.55 g when empty and. What Is The Density Of A Graduated Cylinder.
From www.animalia-life.club
Reading A Graduated Cylinder What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). That means when you read the volume, you can estimate to the hundredths place. What is the density of. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant. What Is The Density Of A Graduated Cylinder.
From microbeonline.com
Graduated Cylinder Types, Uses, and How to Use It • Microbe Online What Is The Density Of A Graduated Cylinder A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Suppose you place 3.31 ml of a substance into a graduated cylinder. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. Calculating density uses the formula d = m ÷. What Is The Density Of A Graduated Cylinder.
From ashleytarolee.blogspot.com
Cross Section of a Graduated Cylinder AshleytaroLee What Is The Density Of A Graduated Cylinder Find mass using a balance scale, and use water displacement to find the volume. What is the density of. Use these values to determine the density of this. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. In addition to measuring liquid volumes, graduated cylinders are used to. What Is The Density Of A Graduated Cylinder.
From www.numerade.com
SOLVED Part 3 Density of a liquid Measure the mass and volume of the What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). What is the density of. A 14.74 g piece of aluminum is added to the water, and. What Is The Density Of A Graduated Cylinder.
From stock.adobe.com
Measuring cylinder. Vector illustration. Cylinder graduated tube vector What Is The Density Of A Graduated Cylinder That means when you read the volume, you can estimate to the hundredths place. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. Suppose you place 3.31 ml of a substance into a graduated cylinder. What is the density of the aluminum in g/ml? A piece of rebar is weighed and then. What Is The Density Of A Graduated Cylinder.
From courses.lumenlearning.com
Measurement Uncertainty, Accuracy, and Precision CHEM 1305 General What Is The Density Of A Graduated Cylinder In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. Find mass using a balance scale, and use water displacement to find the volume. Use these values to determine the density of this. What is the density of the aluminum in g/ml? The graduated cylinder has a mass of 12.55 g when empty. What Is The Density Of A Graduated Cylinder.
From www.slideshare.net
Biology density What Is The Density Of A Graduated Cylinder Find mass using a balance scale, and use water displacement to find the volume. Suppose you place 3.31 ml of a substance into a graduated cylinder. A graduated cylinder contains 10.00 ml water. By measuring the displacement of liquid when an object is. The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g. What Is The Density Of A Graduated Cylinder.
From www.numerade.com
SOLVED(a) To identify a liquid substance, a student determined its What Is The Density Of A Graduated Cylinder Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. What is the density of the aluminum in g/ml? What is the density of. The graduated cylinder has. What Is The Density Of A Graduated Cylinder.
From www.numerade.com
SOLVED A graduated cylinder with a mass of 105.56 g has 45.4 mL of a What Is The Density Of A Graduated Cylinder In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. By measuring the displacement of liquid when an object is. What is the density of the aluminum in g/ml? Calculating density uses the formula d. What Is The Density Of A Graduated Cylinder.
From openbooks.library.umass.edu
Review of Significant Figures Physics 132 Lab Manual What Is The Density Of A Graduated Cylinder Find mass using a balance scale, and use water displacement to find the volume. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. A graduated cylinder contains 10.00 ml water. Computes. What Is The Density Of A Graduated Cylinder.
From www.chegg.com
Measurement Result Mass of 100 mL graduated cylinder What Is The Density Of A Graduated Cylinder A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Find mass using a balance scale, and use water displacement to find the volume. That means when you read the volume, you can estimate to the hundredths place. What is the density of. In addition to measuring liquid volumes, graduated cylinders are. What Is The Density Of A Graduated Cylinder.
From www.chegg.com
Solved Problem 2 Given A graduated cylinder filled with What Is The Density Of A Graduated Cylinder A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. That means when you read the volume, you can estimate to the hundredths place. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). If you look at a 10. What Is The Density Of A Graduated Cylinder.
From www.numerade.com
SOLVED(a) To identify a liquid substance, a student determined its What Is The Density Of A Graduated Cylinder The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. What is the density of the aluminum in g/ml? Suppose you place 3.31 ml of a substance into a graduated cylinder. If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a. What Is The Density Of A Graduated Cylinder.
From worksheetfulljointure.z21.web.core.windows.net
Measuring A Graduated Cylinder What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). That means when you read the volume, you can estimate to the hundredths place. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. Suppose you place 3.31. What Is The Density Of A Graduated Cylinder.
From sciencenotes.org
Table of Density of Common Materials What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. What is the density of. Calculating density uses the formula d = m ÷ v, where d means. What Is The Density Of A Graduated Cylinder.
From www.laboratory-apparatus.com
Measuring Cylinder/Graduated Cylinder Definition Uses Functions All What Is The Density Of A Graduated Cylinder Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). What is the density of the aluminum in g/ml? A graduated cylinder contains 10.00 ml water. By measuring the displacement of liquid when an object is. In addition to measuring liquid volumes, graduated cylinders are used to calculate. What Is The Density Of A Graduated Cylinder.
From www.slideshare.net
Using a Graduated Cylinder What Is The Density Of A Graduated Cylinder Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. By measuring the displacement of liquid when an object is. In addition to measuring liquid volumes, graduated cylinders are used to. What Is The Density Of A Graduated Cylinder.
From www.ck12.org
Q What is the volume of the liquid in the graduated cylinder pictured What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). Find mass using a balance scale, and use water displacement to find the volume. What is the density of the aluminum in g/ml? In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an. What Is The Density Of A Graduated Cylinder.
From www.sliderbase.com
Scientific Measurement Presentation Chemistry What Is The Density Of A Graduated Cylinder A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. That means when you read the volume, you can estimate to the hundredths place. A graduated cylinder contains 10.00 ml water. Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height. What Is The Density Of A Graduated Cylinder.
From www.youtube.com
Calculating density and volume of a cylinder (GCSE maths) YouTube What Is The Density Of A Graduated Cylinder Computes the lateral area (surface area of the sides) of a slanted cylinder based on its radius, height and slant angle (θ). Use these values to determine the density of this. By measuring the displacement of liquid when an object is. What is the density of the aluminum in g/ml? The graduated cylinder has a mass of 12.55 g when. What Is The Density Of A Graduated Cylinder.
From www.youtube.com
Measuring Cylinder Graduated Cylinder Definition Uses Functions etc What Is The Density Of A Graduated Cylinder In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. A. What Is The Density Of A Graduated Cylinder.
From pixmob.info
A Rock Is Placed In A Graduated Cylinder PIXMOB What Is The Density Of A Graduated Cylinder That means when you read the volume, you can estimate to the hundredths place. If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. What is the density of. By measuring the. What Is The Density Of A Graduated Cylinder.
From www.paramhimalaya.com
Experiment To determine the density of solid ( Denser than water ) by What Is The Density Of A Graduated Cylinder What is the density of the aluminum in g/ml? Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. If you look at a 10 ml graduated cylinder, for example, the smallest. What Is The Density Of A Graduated Cylinder.
From www.gradegorilla.com
GCSE Physics Density Grade Gorilla What Is The Density Of A Graduated Cylinder What is the density of the aluminum in g/ml? A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. A graduated cylinder contains 10.00 ml water. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. Calculating density uses the formula. What Is The Density Of A Graduated Cylinder.
From www.e-streetlight.com
Reading Graduated Cylinders Worksheet What Is The Density Of A Graduated Cylinder What is the density of. Use these values to determine the density of this. Suppose you place 3.31 ml of a substance into a graduated cylinder. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. If you look at a 10 ml graduated cylinder, for example, the smallest. What Is The Density Of A Graduated Cylinder.
From drofero2bmaterialdb.z13.web.core.windows.net
Reading A Graduated Cylinder Worksheet What Is The Density Of A Graduated Cylinder Suppose you place 3.31 ml of a substance into a graduated cylinder. In addition to measuring liquid volumes, graduated cylinders are used to calculate the density of an object. What is the density of. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml. The graduated cylinder has a mass of 12.55. What Is The Density Of A Graduated Cylinder.
From www.numerade.com
What is the density (g/ml) of an object that has a mass of 14.01 grams What Is The Density Of A Graduated Cylinder That means when you read the volume, you can estimate to the hundredths place. What is the density of the aluminum in g/ml? The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. By measuring the displacement of liquid when an object is. Use these values to determine the. What Is The Density Of A Graduated Cylinder.
From www.chegg.com
Solved 9) An empty graduated cylinder has a mass of 68.00 What Is The Density Of A Graduated Cylinder The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with. What Is The Density Of A Graduated Cylinder.
From www.scribd.com
Graduated Cylinder Worksheet What Is The Density Of A Graduated Cylinder Use these values to determine the density of this. What is the density of the aluminum in g/ml? A piece of rebar is weighed and then submerged in a graduated cylinder partially filled with water, with results as shown. Suppose you place 3.31 ml of a substance into a graduated cylinder. If you look at a 10 ml graduated cylinder,. What Is The Density Of A Graduated Cylinder.
From www.3bscientific.com
Graduated Cylinder, 100 ml 1002870 U14205 Density and Volume 3B What Is The Density Of A Graduated Cylinder If you look at a 10 ml graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1 ml). The graduated cylinder has a mass of 12.55 g when empty and a mass of 15.57 g after adding the substance. A 14.74 g piece of aluminum is added to the water, and the volume rises to 15.46 ml.. What Is The Density Of A Graduated Cylinder.
From www.youtube.com
How to Calculate the Height of a Cylinder Using the Density Formula What Is The Density Of A Graduated Cylinder Calculating density uses the formula d = m ÷ v, where d means density, m means mass and v means volume. Find mass using a balance scale, and use water displacement to find the volume. Suppose you place 3.31 ml of a substance into a graduated cylinder. A graduated cylinder contains 10.00 ml water. What is the density of. If. What Is The Density Of A Graduated Cylinder.