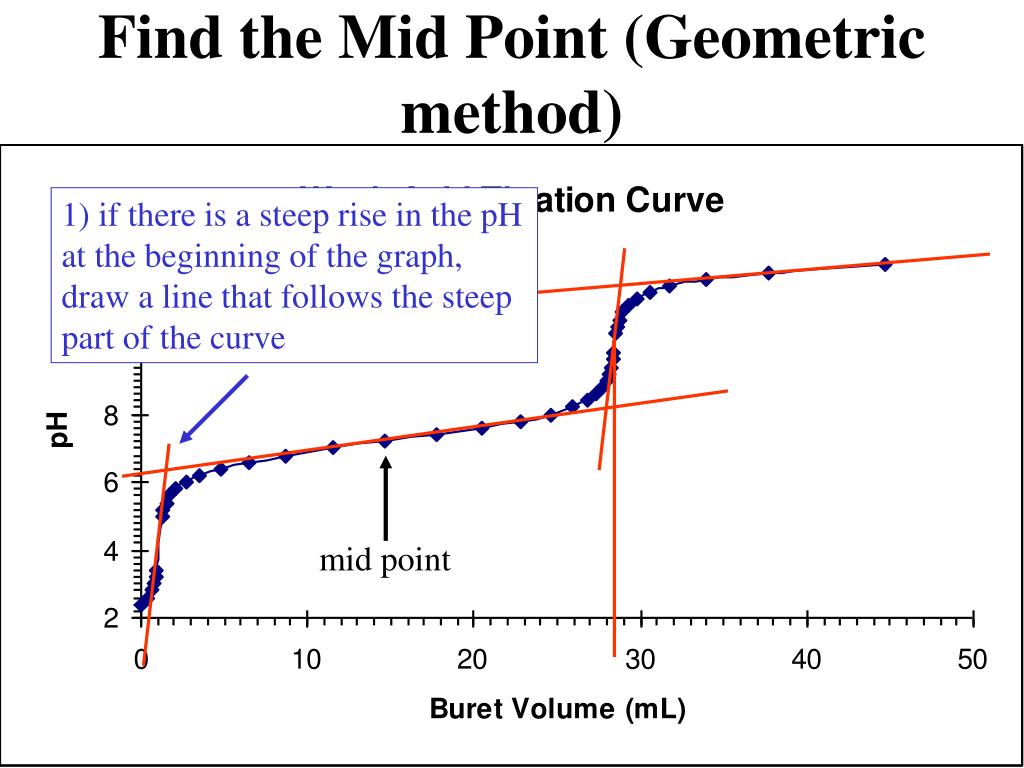
How To Calculate Titration Equivalence Point . Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. By the end of this section, you will be able to: At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. The mmol ch 3 cooh:
from www.slideserve.com
The equivalence point occurs when equal moles of acid react with equal moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. By the end of this section, you will be able to: The mmol ch 3 cooh: This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. At the equivalence point, the number. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
PPT How to Interpret Titration Curves PowerPoint Presentation, free
How To Calculate Titration Equivalence Point To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. The mmol ch 3 cooh: By the end of this section, you will be able to: At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent How To Calculate Titration Equivalence Point The mmol ch 3 cooh: By the end of this section, you will be able to: The equivalence point occurs when equal moles of acid react with equal moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point means the point during titration. How To Calculate Titration Equivalence Point.
From quizrecentring.z21.web.core.windows.net
How To Find Equivalence Point How To Calculate Titration Equivalence Point This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. At the equivalence point, the number. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. Equivalence point means the point during titration at. How To Calculate Titration Equivalence Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube How To Calculate Titration Equivalence Point By the end of this section, you will be able to: At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. This type is usually used in titrations that. How To Calculate Titration Equivalence Point.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby How To Calculate Titration Equivalence Point Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. At the. How To Calculate Titration Equivalence Point.
From www.numerade.com
SOLVEDIn the titration of a weak acid with a strong base, how do you How To Calculate Titration Equivalence Point At the equivalence point, the number. By the end of this section, you will be able to: Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. The equivalence point occurs when equal moles of acid react with equal moles of. How To Calculate Titration Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free How To Calculate Titration Equivalence Point At the equivalence point, the number. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. By the end of this section, you will be able to: This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. The. How To Calculate Titration Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube How To Calculate Titration Equivalence Point \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. At the equivalence point, the number. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point occurs when. How To Calculate Titration Equivalence Point.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. By the end of this section, you will be able to: At the equivalence point, the number. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. Titration calculations at the equivalence point in a neutralization, the. How To Calculate Titration Equivalence Point.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. The mmol ch 3 cooh: The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration. How To Calculate Titration Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co How To Calculate Titration Equivalence Point Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The mmol ch 3 cooh: Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. By the end of this section, you will be able to: This type is usually. How To Calculate Titration Equivalence Point.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there How To Calculate Titration Equivalence Point To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The equivalence point occurs when equal moles of acid react with equal moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme. How To Calculate Titration Equivalence Point.
From studyfurnishers.z4.web.core.windows.net
How To Determine Equivalence Point How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The equivalence point occurs when equal moles of acid react with equal moles of base. By the end of this. How To Calculate Titration Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve How To Calculate Titration Equivalence Point At the equivalence point, the number. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both. How To Calculate Titration Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent How To Calculate Titration Equivalence Point At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation. How To Calculate Titration Equivalence Point.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube How To Calculate Titration Equivalence Point By the end of this section, you will be able to: At the equivalence point, the number. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. The equivalence point occurs when equal moles of acid react with equal moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. To evaluate the relationship. How To Calculate Titration Equivalence Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe How To Calculate Titration Equivalence Point \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The mmol ch 3 cooh: By the end of this section, you will be able to:. How To Calculate Titration Equivalence Point.
From www.numerade.com
SOLVEDWhat is a titration? What is the equivalence point? How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. Calculate ph at the equivalence. How To Calculate Titration Equivalence Point.
From www.youtube.com
Calculating the Equivalence Point YouTube How To Calculate Titration Equivalence Point The equivalence point occurs when equal moles of acid react with equal moles of base. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. At the equivalence point, the number. The determination. How To Calculate Titration Equivalence Point.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube How To Calculate Titration Equivalence Point Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. How To Calculate Titration Equivalence Point.
From www.vrogue.co
Point In Acid Base Titration The Equivalence Point So vrogue.co How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. At the equivalence point, the number. The equivalence point occurs when equal moles of acid react with equal moles of base. To. How To Calculate Titration Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube How To Calculate Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. The equivalence point occurs when equal moles of acid react with equal moles of base. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1. How To Calculate Titration Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube How To Calculate Titration Equivalence Point By the end of this section, you will be able to: At the equivalence point, the number. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. This. How To Calculate Titration Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii How To Calculate Titration Equivalence Point By the end of this section, you will be able to: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The mmol ch 3 cooh: Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. At the. How To Calculate Titration Equivalence Point.
From studymarxianism.z21.web.core.windows.net
How To Find Equivalence Point How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. By the end of this section, you will be able to: The mmol ch 3 cooh: Titration calculations at the equivalence point. How To Calculate Titration Equivalence Point.
From study.com
Finding the Equivalence Point Titration & Examples Lesson How To Calculate Titration Equivalence Point This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. By the end of this section, you will be able to: The mmol ch 3 cooh: \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25.. How To Calculate Titration Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 How To Calculate Titration Equivalence Point The equivalence point occurs when equal moles of acid react with equal moles of base. At the equivalence point, the number. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. To. How To Calculate Titration Equivalence Point.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph How To Calculate Titration Equivalence Point \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. At the equivalence point, the number. The mmol ch 3 cooh: Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate. How To Calculate Titration Equivalence Point.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID234018 How To Calculate Titration Equivalence Point At the equivalence point, the number. The mmol ch 3 cooh: By the end of this section, you will be able to: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to. How To Calculate Titration Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. \(15 ml ch_{3}cooh * \dfrac{.15 mmol ch_{3}cooh}{1 ml} =2.25. This type is usually. How To Calculate Titration Equivalence Point.
From www.youtube.com
How to Calculate the Volume of Titrant Needed to Reach Equivalence How To Calculate Titration Equivalence Point Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The mmol ch 3 cooh: The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. Equivalence point means the point during. How To Calculate Titration Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point How To Calculate Titration Equivalence Point The equivalence point occurs when equal moles of acid react with equal moles of base. The mmol ch 3 cooh: By the end of this section, you will be able to: This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding. At the equivalence point, the number. Equivalence point means the point during titration at. How To Calculate Titration Equivalence Point.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube How To Calculate Titration Equivalence Point The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Calculate ph at the equivalence point of formic acid. How To Calculate Titration Equivalence Point.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. By the end of this section, you will be able to: Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This type is usually used in titrations that involve biochemical reactions. How To Calculate Titration Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube How To Calculate Titration Equivalence Point The equivalence point occurs when equal moles of acid react with equal moles of base. The mmol ch 3 cooh: Calculate ph at the equivalence point of formic acid titration with naoh, assuming both titrant and titrated acid concentrations are. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by. How To Calculate Titration Equivalence Point.
From www.coursehero.com
[Solved] Calculate the pH at the equivalence point for the titration How To Calculate Titration Equivalence Point Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by. How To Calculate Titration Equivalence Point.