What Is E2B R2 . It was revised in 2005 and. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case.
from www.slideshare.net
It was revised in 2005 and. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in.
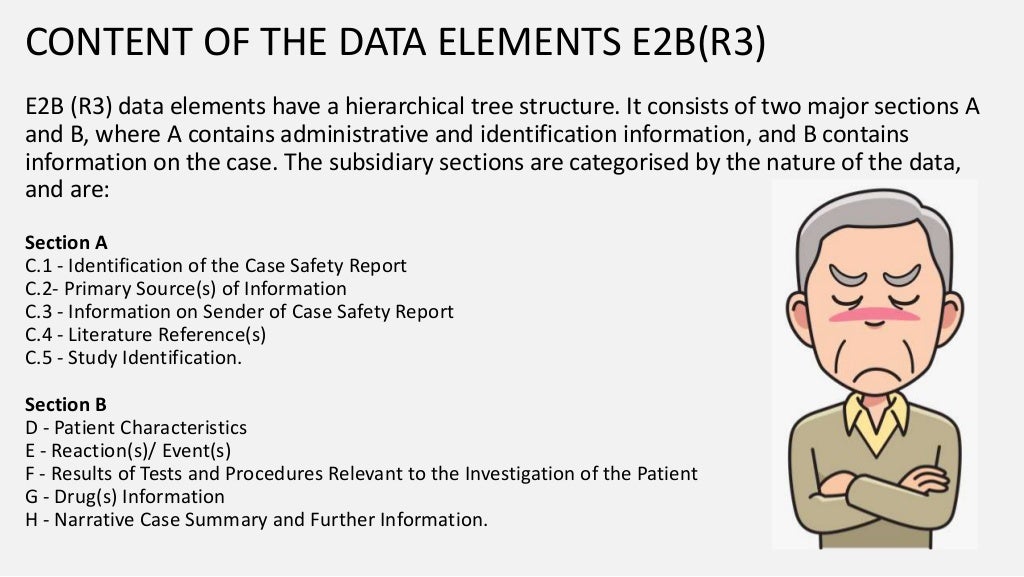
E2B(R2) vs E2B(R3) ICSR ELEMENTS
What Is E2B R2 eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. It was revised in 2005 and.
From www.studocu.com
R2 Guideline DAE R2 Guidelines ACADEMIC YEAR 2022 Professor What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. It was revised in. What Is E2B R2.
From www.slideserve.com
PPT Ebusiness en de nieuwe farmacovigilantie wetgeving PowerPoint What Is E2B R2 this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. eudravigilance is a. What Is E2B R2.
From www.slideshare.net
What's E2B Viewer? What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. ich. What Is E2B R2.
From support.veeva.com
Why the Case Identifier(s) Field is not populated on the Inbox Item What Is E2B R2 this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. It was revised in 2005 and. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. . What Is E2B R2.
From www.youtube.com
ICH E2B (R2 e R3) RDC406 ANVISA VIGIMED DRM YouTube What Is E2B R2 It was revised in 2005 and. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. this document is a harmonized tripartite guideline for the data elements of case safety. What Is E2B R2.
From www.slideserve.com
PPT Ebusiness en de nieuwe farmacovigilantie wetgeving PowerPoint What Is E2B R2 ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. It was revised in 2005 and. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european.. What Is E2B R2.
From www.researchgate.net
Items identified by reviewing the ICH E2A and E2B(R3) guidelines, along What Is E2B R2 ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. this document provides. What Is E2B R2.
From blogs.perficient.com
What Is The Difference Between E2B(R2) And E2B(R3)? What Is E2B R2 e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. It was revised. What Is E2B R2.
From dokumen.tips
(DOC) GUIDELINE FOR GOOD CLINICAL PRACTICEpeople.duke.edu/ghturner/ICH What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). eudravigilance is a. What Is E2B R2.
From hxelbuotd.blob.core.windows.net
What Is E2B Reporting at William Pierro blog What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. It was revised in 2005 and. . What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS PPT What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. It was revised in 2005 and. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical. What Is E2B R2.
From www.linkedin.com
E2B and E2b(R2) and E2b(R3) The Variations What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. It was revised in 2005 and. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical. What Is E2B R2.
From www.youtube.com
ICH ICSR E2B R3 Guideline How to Fill ICSR What Data Needed in What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document provides the implementation guide. What Is E2B R2.
From hxelbuotd.blob.core.windows.net
What Is E2B Reporting at William Pierro blog What Is E2B R2 It was revised in 2005 and. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. . What Is E2B R2.
From www.linkedin.com
Comparison, Advantages and Limitations of ICSR E2B (R2) and E2B (R3) What Is E2B R2 eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. this document is a harmonized. What Is E2B R2.
From dokumen.tips
(PDF) ICH E2B (R3) 日本製薬工業協会 E2B(R3)とM2の改訂 E2B (R2) 個別症例安全性報告を伝送するための What Is E2B R2 this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. It was revised in 2005 and. this document is a harmonized tripartite guideline for the data elements of case safety. What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS PPT What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. It was revised in. What Is E2B R2.
From www.youtube.com
ICH E2B R2 to R3 conversion YouTube What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. It. What Is E2B R2.
From blog.drugvigil.com
🥨 E2B and E2B(R2) and E2B(R3) The Variations Drugvigil's Blog What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. ich e2b (r3) is. What Is E2B R2.
From www.pdffiller.com
Fillable Online Comparison, Advantages and Limitations of ICSR E2B (R2 What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document provides the. What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B R2 ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). It was revised in 2005 and. learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case.. What Is E2B R2.
From fyodujhox.blob.core.windows.net
What Is An E2B Report at Kevin Gregory blog What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. It was revised in 2005 and. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of. What Is E2B R2.
From www.studocu.com
E2B R2 Guideline INTERNATIONAL CONFERENCE ON HARMONISATION OF What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. ich e2b (r3) is a guideline. What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. e2b r2. What Is E2B R2.
From fyodujhox.blob.core.windows.net
What Is An E2B Report at Kevin Gregory blog What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. It was revised in 2005 and. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory. What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B R2 It was revised in 2005 and. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory. What Is E2B R2.
From www.slideserve.com
PPT Ebusiness en de nieuwe farmacovigilantie wetgeving PowerPoint What Is E2B R2 It was revised in 2005 and. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical. What Is E2B R2.
From www.sophossystem.com
Sophos eSynchronize E2B R3 Solution Sophos IT Services What Is E2B R2 eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. learn how to implement. What Is E2B R2.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance. What Is E2B R2.
From dokumen.tips
(PDF) Oracle Safety Cloud Infographic · E2B(R2), eVAERS, eMDR(R2), MIR What Is E2B R2 this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). . What Is E2B R2.
From kufactor.ru
ICH E2B GUIDELINES PDF What Is E2B R2 e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. It was revised in 2005 and. . What Is E2B R2.
From hxelbuotd.blob.core.windows.net
What Is E2B Reporting at William Pierro blog What Is E2B R2 e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. this document is a harmonized tripartite guideline for the data elements of case safety reports transmitted to regulatory authorities. . What Is E2B R2.
From cloudfoundation.com
What is and What is used for? CloudFoundation What Is E2B R2 learn how to implement the ich standard for electronic transmission of individual case safety reports (icsrs) in. ich e2b (r3) is a guideline on clinical safety data management for individual case safety reports (icsrs). e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. eudravigilance is. What Is E2B R2.
From www.vitrana.com
TransformPV E2B R2 E2B R3 Vitrana What Is E2B R2 e2b (r3) over e2b (r2) is an option for inclusiveness & collaboration with other stakeholders by addition of enhanced data. It was revised in 2005 and. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. this document is a harmonized tripartite guideline for the data elements of case safety reports. What Is E2B R2.
From safety.veevavault.help
Enable Receiver Information Export for E2B(R2) & PMDA E2B(R3) Vault Help What Is E2B R2 this document provides the implementation guide for the ich guideline e2b(r3) on electronic transmission of individual case. e2b r2 is a data standard for transmitting individual case safety reports (icsrs) in clinical trials. eudravigilance is a system for reporting and evaluating suspected adverse reactions to medicinal products in the european. ich e2b (r3) is a guideline. What Is E2B R2.