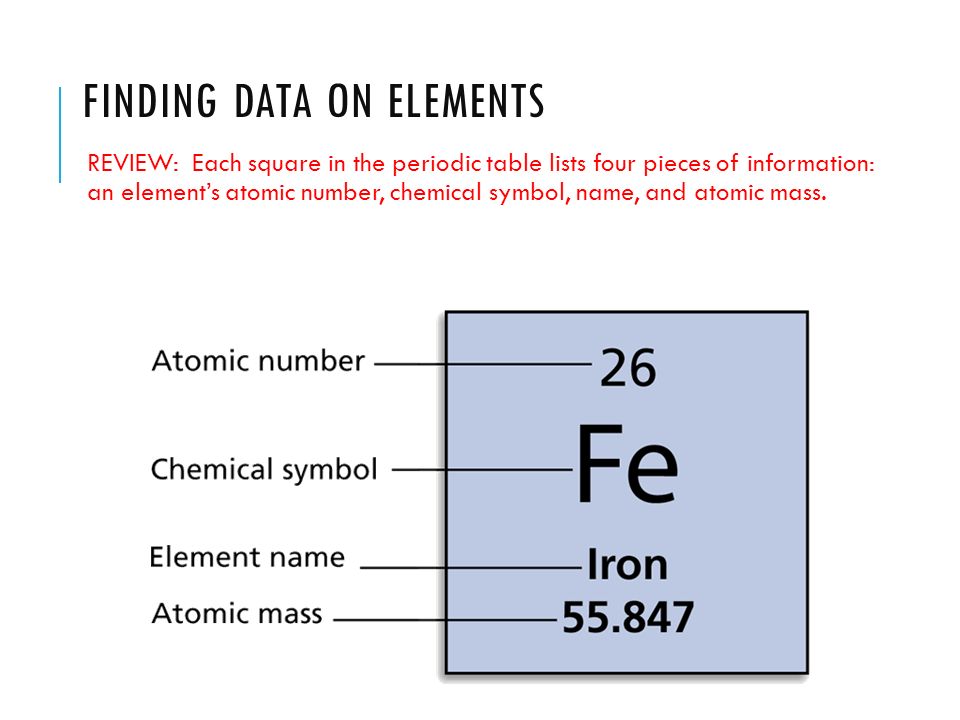
How To Calculate The Mass Number Of A Element . In physics the mass number is indicated with an a, and varies. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. It represents the mass of. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number = number of neutrons + atomic number. Give the symbol of each isotope with the mass number as the superscript and. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). The mass number is the total number of protons and neutrons present inside the nucleus of an atom. The mass number is defined as the total number of protons and neutrons in an atom. Neutrons are the subatomic particles present in atomic nuclei like protons. It's important to remember that mass number is not the same as atomic mass. Have you ever wondered about the mass of a single atom?
from periodictable.me
The mass number = number of neutrons + atomic number. It's important to remember that mass number is not the same as atomic mass. Give the symbol of each isotope with the mass number as the superscript and. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. It represents the mass of. Have you ever wondered about the mass of a single atom? The mass number is defined as the total number of protons and neutrons in an atom. In physics the mass number is indicated with an a, and varies.
How To Find Element Atomic Number, Element Name & Symbol
How To Calculate The Mass Number Of A Element The mass number is the total number of protons and neutrons present inside the nucleus of an atom. Give the symbol of each isotope with the mass number as the superscript and. Neutrons are the subatomic particles present in atomic nuclei like protons. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). The mass number is defined as the total number of protons and neutrons in an atom. The mass number is the total number of protons and neutrons present inside the nucleus of an atom. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Have you ever wondered about the mass of a single atom? It represents the mass of. The mass number = number of neutrons + atomic number. In physics the mass number is indicated with an a, and varies. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. It's important to remember that mass number is not the same as atomic mass.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps How To Calculate The Mass Number Of A Element The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). In physics the mass number is indicated with an a, and varies. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Have you ever wondered about the. How To Calculate The Mass Number Of A Element.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number How To Calculate The Mass Number Of A Element It's important to remember that mass number is not the same as atomic mass. The mass number is the total number of protons and neutrons present inside the nucleus of an atom. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). The mass number = number of. How To Calculate The Mass Number Of A Element.
From periodictable.me
What is Sodium Mass Number and Atomic Number? How To Calculate The Mass Number Of A Element In physics the mass number is indicated with an a, and varies. Give the symbol of each isotope with the mass number as the superscript and. It represents the mass of. The mass number is defined as the total number of protons and neutrons in an atom. It's important to remember that mass number is not the same as atomic. How To Calculate The Mass Number Of A Element.
From www.youtube.com
CHEMISTRY 101 Dimensional Analysis Mass of compound to mass of How To Calculate The Mass Number Of A Element Give the symbol of each isotope with the mass number as the superscript and. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. In physics the mass number is indicated with an a, and varies. The mass number is defined as the total number of protons and neutrons in. How To Calculate The Mass Number Of A Element.
From www.wikihow.com
How to Find Average Atomic Mass StepbyStep Calculation How To Calculate The Mass Number Of A Element It represents the mass of. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. In physics the mass number is indicated with an a, and varies. Have you ever wondered about. How To Calculate The Mass Number Of A Element.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4825731 How To Calculate The Mass Number Of A Element The mass number is the total number of protons and neutrons present inside the nucleus of an atom. Have you ever wondered about the mass of a single atom? It's important to remember that mass number is not the same as atomic mass. The molar mass of an element is found on the periodic table, and it is the element's. How To Calculate The Mass Number Of A Element.
From periodictable.me
How To Find Element Atomic Number, Element Name & Symbol How To Calculate The Mass Number Of A Element The mass number = number of neutrons + atomic number. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. In physics the mass number is indicated with an a, and varies. It's important to remember. How To Calculate The Mass Number Of A Element.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of How To Calculate The Mass Number Of A Element The mass number is defined as the total number of protons and neutrons in an atom. Give the symbol of each isotope with the mass number as the superscript and. In physics the mass number is indicated with an a, and varies. The mass number = number of neutrons + atomic number. It's important to remember that mass number is. How To Calculate The Mass Number Of A Element.
From www.youtube.com
How To Calculate The Average Atomic Mass YouTube How To Calculate The Mass Number Of A Element It represents the mass of. In physics the mass number is indicated with an a, and varies. Give the symbol of each isotope with the mass number as the superscript and. Neutrons are the subatomic particles present in atomic nuclei like protons. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass. How To Calculate The Mass Number Of A Element.
From sciencenotes.org
Mass Number Versus Atomic Number and Atomic Mass How To Calculate The Mass Number Of A Element The mass number = number of neutrons + atomic number. In physics the mass number is indicated with an a, and varies. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in. How To Calculate The Mass Number Of A Element.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples How To Calculate The Mass Number Of A Element Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Give the symbol of each isotope with the mass number as the superscript and. Have you ever wondered about the mass of a single. How To Calculate The Mass Number Of A Element.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number How To Calculate The Mass Number Of A Element Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number is defined as the total number of protons and neutrons in an atom. It's important to remember that mass number is not the same as atomic mass. Neutrons are the subatomic particles present in atomic nuclei like. How To Calculate The Mass Number Of A Element.
From periodictable.me
Way to Find Atomic Mass of Elements with Examples How To Calculate The Mass Number Of A Element The mass number is the total number of protons and neutrons present inside the nucleus of an atom. The mass number is defined as the total number of protons and neutrons in an atom. Give the symbol of each isotope with the mass number as the superscript and. The molar mass of an element is found on the periodic table,. How To Calculate The Mass Number Of A Element.
From elchoroukhost.net
The Periodic Table Of Elements With Names And Symbols Atomic Mass How To Calculate The Mass Number Of A Element Neutrons are the subatomic particles present in atomic nuclei like protons. Give the symbol of each isotope with the mass number as the superscript and. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. The mass number is the total number of protons and neutrons present inside the nucleus of an. How To Calculate The Mass Number Of A Element.
From www.sliderbase.com
Atomic Number How To Calculate The Mass Number Of A Element Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. It represents the mass of. The molar mass of an element is found on the periodic table, and it is the element's. How To Calculate The Mass Number Of A Element.
From www.pinterest.com
The mass number, A, of an element is the total number of protons and How To Calculate The Mass Number Of A Element Neutrons are the subatomic particles present in atomic nuclei like protons. In physics the mass number is indicated with an a, and varies. It represents the mass of. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Calculate the mass of a single atom of any element by dividing the atomic mass by. How To Calculate The Mass Number Of A Element.
From sciencenotes.org
Use Avogadro's Number to Calculate the Mass of a Single Atom How To Calculate The Mass Number Of A Element Give the symbol of each isotope with the mass number as the superscript and. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). In physics the mass number is indicated with an a, and varies. Neutrons are the subatomic particles present in atomic nuclei like protons. The. How To Calculate The Mass Number Of A Element.
From www.sliderbase.com
The Mole Presentation Chemistry How To Calculate The Mass Number Of A Element It represents the mass of. The mass number is defined as the total number of protons and neutrons in an atom. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number. How To Calculate The Mass Number Of A Element.
From periodictable.me
How To Find Element Atomic Number, Element Name & Symbol How To Calculate The Mass Number Of A Element It represents the mass of. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). Have you ever wondered about the mass of a single atom? In physics the mass number is indicated with an a, and varies. The mass number = number of neutrons + atomic number.. How To Calculate The Mass Number Of A Element.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number How To Calculate The Mass Number Of A Element The mass number = number of neutrons + atomic number. The mass number is the total number of protons and neutrons present inside the nucleus of an atom. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). Mass number is an integer equal to the sum of. How To Calculate The Mass Number Of A Element.
From www.youtube.com
Mass Number Definition, Equation and Examples Chemistry YouTube How To Calculate The Mass Number Of A Element The mass number is the total number of protons and neutrons present inside the nucleus of an atom. Neutrons are the subatomic particles present in atomic nuclei like protons. Have you ever wondered about the mass of a single atom? Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. In physics the mass. How To Calculate The Mass Number Of A Element.
From www.slideserve.com
PPT Honors Biology Chapter 2 PowerPoint Presentation, free download How To Calculate The Mass Number Of A Element The mass number = number of neutrons + atomic number. It's important to remember that mass number is not the same as atomic mass. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Give the symbol of each isotope with the mass number as the superscript and. Calculate the mass of a single. How To Calculate The Mass Number Of A Element.
From quizparaguayan.z4.web.core.windows.net
How To Calculate The Molecular Mass How To Calculate The Mass Number Of A Element Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number = number of neutrons + atomic number. The mass number is defined as the total number of protons and. How To Calculate The Mass Number Of A Element.
From atomic-zz.blogspot.com
24 TUTORIAL PERIODIC TABLE CALCULATE ATOMIC MASS WITH PDF AND VIDEO How To Calculate The Mass Number Of A Element It's important to remember that mass number is not the same as atomic mass. Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. Have you ever wondered about the mass of a single atom? Calculate the mass number of each isotope by adding together the numbers of protons and. How To Calculate The Mass Number Of A Element.
From courses.lumenlearning.com
The Periodic Table of Elements Biology for Majors I How To Calculate The Mass Number Of A Element The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). In physics the mass number is indicated with an a, and varies. Give the symbol of each isotope with the mass number as the superscript and. The mass number is the total number of protons and neutrons present. How To Calculate The Mass Number Of A Element.
From periodictable.me
Way to Find Atomic Mass of Elements with Examples How To Calculate The Mass Number Of A Element Have you ever wondered about the mass of a single atom? Mass number is an integer equal to the sum of the number of protons and neutrons of an atomic nucleus. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). The mass number = number of neutrons. How To Calculate The Mass Number Of A Element.
From www.youtube.com
Chem121 Atomic Number and Mass Number 3 4 YouTube How To Calculate The Mass Number Of A Element The mass number = number of neutrons + atomic number. Give the symbol of each isotope with the mass number as the superscript and. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in. How To Calculate The Mass Number Of A Element.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow How To Calculate The Mass Number Of A Element It's important to remember that mass number is not the same as atomic mass. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). Have you ever wondered about the mass of a single atom? The mass number is defined as the total number of protons and neutrons. How To Calculate The Mass Number Of A Element.
From newtondesk.com
Explain Atomic Number And Mass Number of Element Chemistry How To Calculate The Mass Number Of A Element Calculate the mass of a single atom of any element by dividing the atomic mass by avogadro’s number. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number = number of neutrons + atomic number. Have you ever wondered about the mass of a single atom? The mass number is the. How To Calculate The Mass Number Of A Element.
From www.youtube.com
Atomic Number and Mass Number.mov YouTube How To Calculate The Mass Number Of A Element Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number = number of neutrons + atomic number. Give the symbol of each isotope with the mass number as the superscript and. Have you ever wondered about the mass of a single atom? Calculate the mass of a single atom of any. How To Calculate The Mass Number Of A Element.
From www.ck12.org
Mass Number Example 1 ( Video ) Chemistry CK12 Foundation How To Calculate The Mass Number Of A Element Neutrons are the subatomic particles present in atomic nuclei like protons. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). It's important to remember that mass number is not the same as atomic mass. The mass number is defined as the total number of protons and neutrons. How To Calculate The Mass Number Of A Element.
From www.youtube.com
How to calculate the atomic mass of an element when give the atomic How To Calculate The Mass Number Of A Element Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number is the total number of protons and neutrons present inside the nucleus of an atom. Have you ever wondered about the mass of a single atom? In physics the mass number is indicated with an a, and varies. It's important to. How To Calculate The Mass Number Of A Element.
From www.animalia-life.club
Modern Periodic Table With Atomic Mass And Atomic Number How To Calculate The Mass Number Of A Element The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). In physics the mass number is indicated with an a, and varies. Neutrons are the subatomic particles present in atomic nuclei like protons. The mass number = number of neutrons + atomic number. Give the symbol of each. How To Calculate The Mass Number Of A Element.
From www.youtube.com
Periodic Table Atomic Mass Trick To Learn Atomic Mass Number YouTube How To Calculate The Mass Number Of A Element Give the symbol of each isotope with the mass number as the superscript and. The mass number is defined as the total number of protons and neutrons in an atom. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number = number of neutrons + atomic number. It's important to remember. How To Calculate The Mass Number Of A Element.
From classnotes.ng
Atomic Number, Mass Number, Isotopes And Calculations ClassNotes.ng How To Calculate The Mass Number Of A Element It's important to remember that mass number is not the same as atomic mass. The mass number is defined as the total number of protons and neutrons in an atom. Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. The mass number = number of neutrons + atomic number. Calculate the mass of. How To Calculate The Mass Number Of A Element.