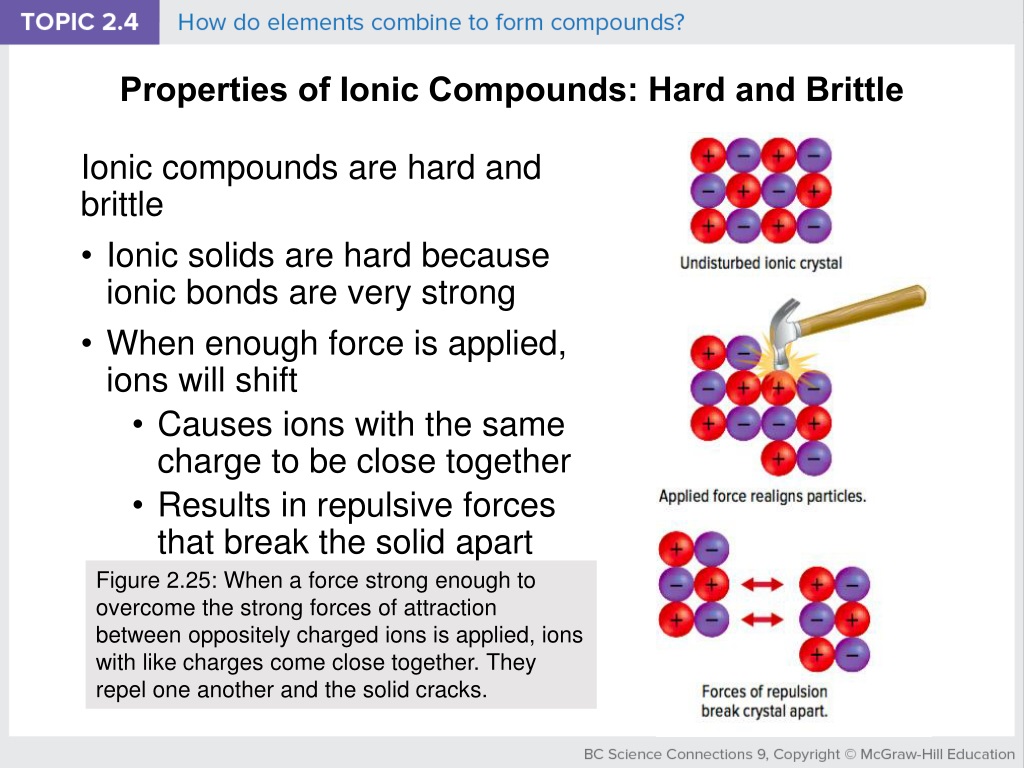
Ionic Compounds Are Brittle . The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds generally have high melting points and high boiling points. A hard material will tend to assume its shape when stressed by an external force. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. The repulsion causes the crystal to shatter along a smooth plane of the crystal. A mechanical force, such as a strike with a hammer, shifts layers of ions. This brings ions with the same charge close to each other. Ionic compounds have many physical properties in common: Ionic compounds are hard and brittle. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are generally hard and brittle. They usually have melting points and boiling points well above room temperature. Ionic compounds have high melting points.
from www.slideserve.com
They usually have melting points and boiling points well above room temperature. Ionic compounds exhibit the property of hardness. Ionic compounds have many physical properties in common: Ionic compounds dissociate into ions when dissolved in water. This brings ions with the same charge close to each other. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. A hard material will tend to assume its shape when stressed by an external force. The repulsion causes the crystal to shatter along a smooth plane of the crystal. Ionic compounds are hard and brittle. Ionic compounds are generally hard and brittle.
PPT BC Science Connections 9 Unit 2 The electron arrangement of
Ionic Compounds Are Brittle They usually have melting points and boiling points well above room temperature. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds have many physical properties in common: Ionic compounds generally have high melting points and high boiling points. A hard material will tend to assume its shape when stressed by an external force. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting points. Ionic compounds exhibit the property of hardness. The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds are hard and brittle. Ionic compounds are generally hard and brittle. They usually have melting points and boiling points well above room temperature. Ionic compounds are hard and brittle. This brings ions with the same charge close to each other. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. A mechanical force, such as a strike with a hammer, shifts layers of ions.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Ionic Compounds Are Brittle Ionic compounds are generally hard and brittle. Ionic compounds have high melting points. Ionic compounds have many physical properties in common: The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds dissociate into ions when dissolved in water. This brings ions with the same charge close to each other. Solutions of ionic compounds and melted ionic. Ionic Compounds Are Brittle.
From slideplayer.com
WRITING AND NAMING IONIC COMPOUNDS ppt download Ionic Compounds Are Brittle Ionic compounds dissociate into ions when dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds generally have high melting points and high boiling points. Ionic compounds have many physical properties in common: This brings ions with the same charge close to each other. Ionic compounds have high melting points.. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Ionic Compounds Are Brittle The stability of ionic crystals makes them hard, but they are brittle. A mechanical force, such as a strike with a hammer, shifts layers of ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. A hard material will tend to assume its shape when stressed by an external force. Ionic compounds exhibit the. Ionic Compounds Are Brittle.
From www.studocu.com
Ionic bonds ionic bonds brittle substances with high melting points Ionic Compounds Are Brittle They usually have melting points and boiling points well above room temperature. Ionic compounds are hard and brittle. The stability of ionic crystals makes them hard, but they are brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have many physical properties. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic and Covalent Compounds ppt download Ionic Compounds Are Brittle Ionic compounds have high melting points. Ionic compounds generally have high melting points and high boiling points. They usually have melting points and boiling points well above room temperature. The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID5979988 Ionic Compounds Are Brittle Ionic compounds have many physical properties in common: Ionic compounds dissociate into ions when dissolved in water. A mechanical force, such as a strike with a hammer, shifts layers of ions. They usually have melting points and boiling points well above room temperature. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make. Ionic Compounds Are Brittle.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Compounds Are Brittle Ionic compounds have many physical properties in common: The stability of ionic crystals makes them hard, but they are brittle. They usually have melting points and boiling points well above room temperature. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds exhibit the property of hardness. A hard material will tend to. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bonding. ppt download Ionic Compounds Are Brittle Ionic compounds are hard and brittle. A hard material will tend to assume its shape when stressed by an external force. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds exhibit the property of hardness. This brings ions with the same charge close to each other. Ionic compounds have many physical properties. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Ionic Compounds Are Brittle This brings ions with the same charge close to each other. The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard and brittle. Ionic compounds have many physical properties in common: The repulsion causes the crystal to shatter along a smooth plane of the crystal. They. Ionic Compounds Are Brittle.
From slideplayer.com
Chapter 6 Section 1 Compounds and Molecules ppt download Ionic Compounds Are Brittle A hard material will tend to assume its shape when stressed by an external force. Ionic compounds have many physical properties in common: Ionic compounds are generally hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. They usually have melting points and boiling points well above room temperature. Ionic compounds are. Ionic Compounds Are Brittle.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Ionic Compounds Are Brittle A hard material will tend to assume its shape when stressed by an external force. This brings ions with the same charge close to each other. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. The repulsion causes the crystal to shatter along a smooth plane of the crystal. Solutions of ionic compounds and. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Ionic Compounds Are Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. They usually have melting points and boiling points well above room temperature. The repulsion causes the crystal to shatter along a smooth plane of the crystal. Ionic compounds have high melting points. The stability of ionic crystals makes them hard,. Ionic Compounds Are Brittle.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Compounds Are Brittle Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic compounds are generally hard and brittle. Ionic compounds generally have high melting points and high boiling points. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. The repulsion causes the crystal to shatter along a smooth. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic and Metallic Bonds ppt download Ionic Compounds Are Brittle Ionic compounds generally have high melting points and high boiling points. Ionic compounds have high melting points. Ionic compounds exhibit the property of hardness. This brings ions with the same charge close to each other. Ionic compounds have many physical properties in common: A mechanical force, such as a strike with a hammer, shifts layers of ions. A hard material. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic bonding. ppt download Ionic Compounds Are Brittle A hard material will tend to assume its shape when stressed by an external force. Ionic compounds are hard and brittle. Ionic compounds have high melting points. The repulsion causes the crystal to shatter along a smooth plane of the crystal. Ionic compounds are hard and brittle. This brings ions with the same charge close to each other. Solutions of. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Ionic Compounds Are Brittle Ionic compounds are generally hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. This brings ions with the same charge close to each other. A hard material will tend to assume its shape when stressed by an external force. They usually have melting points and. Ionic Compounds Are Brittle.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Ionic Compounds Are Brittle This brings ions with the same charge close to each other. A hard material will tend to assume its shape when stressed by an external force. Ionic compounds are hard and brittle. Ionic compounds exhibit the property of hardness. Ionic compounds are generally hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT BC Science Connections 9 Unit 2 The electron arrangement of Ionic Compounds Are Brittle This brings ions with the same charge close to each other. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. Ionic compounds have many physical properties in common: They usually have melting points and boiling points well above room temperature. Ionic compounds generally have high melting points and. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Are Brittle Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. A hard material will tend to assume its shape when stressed by an external force. Ionic compounds dissociate into ions when dissolved in water. This brings ions with the same charge close to each other. The repulsion causes the crystal to shatter along a smooth. Ionic Compounds Are Brittle.
From slideplayer.com
Structure and Properties of Ionic and Covalent Compounds ppt download Ionic Compounds Are Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds generally have high melting points and high boiling points. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds exhibit the property of hardness. The stability of ionic crystals makes them. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Ionic Compounds Are Brittle Ionic compounds are hard and brittle. They usually have melting points and boiling points well above room temperature. A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds have high melting points. The repulsion causes the crystal to shatter. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bonds and Compounds ppt download Ionic Compounds Are Brittle This brings ions with the same charge close to each other. A hard material will tend to assume its shape when stressed by an external force. Ionic compounds are hard and brittle. They usually have melting points and boiling points well above room temperature. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are brittle due to the. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Compounds Chapter ppt download Ionic Compounds Are Brittle Ionic compounds exhibit the property of hardness. This brings ions with the same charge close to each other. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds are hard and brittle. The stability of ionic crystals makes them. Ionic Compounds Are Brittle.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Ionic Compounds Are Brittle This brings ions with the same charge close to each other. They usually have melting points and boiling points well above room temperature. Ionic compounds exhibit the property of hardness. Ionic compounds have many physical properties in common: Ionic compounds generally have high melting points and high boiling points. Ionic compounds are brittle due to the strong bond between the. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID1083550 Ionic Compounds Are Brittle A hard material will tend to assume its shape when stressed by an external force. Ionic compounds have high melting points. This brings ions with the same charge close to each other. A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds are hard and brittle. Ionic compounds exhibit the property of hardness. The. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bonding/Naming. ppt download Ionic Compounds Are Brittle They usually have melting points and boiling points well above room temperature. This brings ions with the same charge close to each other. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic compounds have many physical properties in common: A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds exhibit. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation, free Ionic Compounds Are Brittle Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds are hard and brittle. The repulsion causes the crystal to shatter along a smooth plane of the crystal. Ionic compounds generally have high melting points and high boiling points. Ionic compounds have many physical properties in common: A. Ionic Compounds Are Brittle.
From www.youtube.com
Why ionic solids are brittle? YouTube Ionic Compounds Are Brittle Ionic compounds have many physical properties in common: This brings ions with the same charge close to each other. Ionic compounds generally have high melting points and high boiling points. The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds are hard and brittle. Ionic compounds are generally hard and brittle. Ionic compounds dissociate into ions. Ionic Compounds Are Brittle.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Ionic Compounds Are Brittle Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. They usually have melting points and boiling points well above room temperature. Ionic compounds exhibit the property of hardness. The stability of ionic crystals makes them hard, but they are brittle. The repulsion causes the crystal to shatter along a smooth plane of the crystal.. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic Bonds. ppt download Ionic Compounds Are Brittle Ionic compounds are hard and brittle. Ionic compounds have high melting points. Ionic compounds exhibit the property of hardness. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds generally have high melting points and high boiling points. Ionic compounds have many physical properties. Ionic Compounds Are Brittle.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Ionic Compounds Are Brittle Ionic compounds have high melting points. Ionic compounds are hard and brittle. A mechanical force, such as a strike with a hammer, shifts layers of ions. They usually have melting points and boiling points well above room temperature. The stability of ionic crystals makes them hard, but they are brittle. Ionic compounds generally have high melting points and high boiling. Ionic Compounds Are Brittle.
From www.slideshare.net
Ionic Bonding Notes Ionic Compounds Are Brittle A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds are hard and brittle. Ionic compounds generally have high melting points and high boiling points. Ionic compounds are brittle due to the strong bond between the positive and negative ions that make up the molecules. Ionic compounds have many physical properties in common: The. Ionic Compounds Are Brittle.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Ionic Compounds Are Brittle Ionic compounds have high melting points. A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds generally have high melting points and high boiling points. Ionic compounds exhibit the property of hardness. They usually have melting points and boiling points well above room temperature. This brings ions with the same charge close to each. Ionic Compounds Are Brittle.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Ionic Compounds Are Brittle A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds generally have high melting points and high boiling points. The stability of ionic crystals makes them hard, but they are brittle. The repulsion causes the crystal to shatter along a smooth plane of the crystal.. Ionic Compounds Are Brittle.
From slideplayer.com
Ionic and Covalent Bonds ppt download Ionic Compounds Are Brittle A mechanical force, such as a strike with a hammer, shifts layers of ions. Ionic compounds have high melting points. Ionic compounds generally have high melting points and high boiling points. This brings ions with the same charge close to each other. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds are hard and brittle. Ionic compounds are. Ionic Compounds Are Brittle.