What Are Fuel Cells Give Example . a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or need recharging. In short, they convert the chemical energy of fuels,. They produce electricity and heat as long as fuel is supplied. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion.
from www.vedantu.com
a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. They produce electricity and heat as long as fuel is supplied. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In short, they convert the chemical energy of fuels,. fuel cells work like batteries, but they do not run down or need recharging. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity.
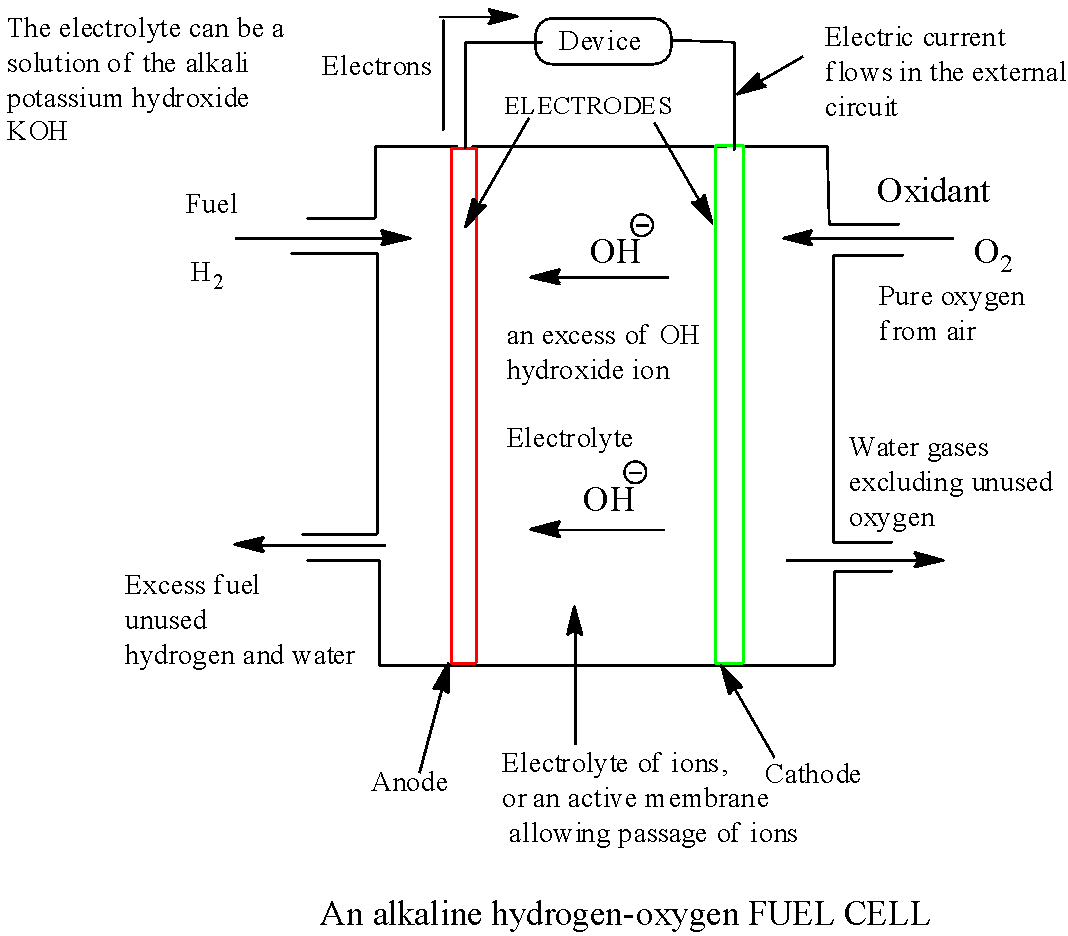
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the
What Are Fuel Cells Give Example fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. They produce electricity and heat as long as fuel is supplied. In a fuel cell, hydrogen and oxygen are combined to generate. In short, they convert the chemical energy of fuels,. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. In short, they convert the chemical energy of fuels,. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical. What Are Fuel Cells Give Example.
From www.tes.com
Fuel Cells GCSE AQA Teaching Resources What Are Fuel Cells Give Example fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. In a fuel cell, hydrogen and oxygen are combined to generate. In. What Are Fuel Cells Give Example.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells work like batteries, but they do not run down or need recharging. In short, they convert the chemical energy of fuels,. They. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 What Are Fuel Cells Give Example a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. They produce electricity and heat. What Are Fuel Cells Give Example.
From www.youtube.com
Introduction to Types of Fuel Cells YouTube What Are Fuel Cells Give Example fuel cells work like batteries, but they do not run down or need recharging. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell. What Are Fuel Cells Give Example.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy What Are Fuel Cells Give Example In a fuel cell, hydrogen and oxygen are combined to generate. In short, they convert the chemical energy of fuels,. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel cells work like batteries, but they do not run down or need recharging.. What Are Fuel Cells Give Example.
From www.geeksforgeeks.org
Fuel Cells Definition, Types, Advantages, Limitations What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In short, they convert the chemical energy of fuels,. fuel. What Are Fuel Cells Give Example.
From electricala2z.com
Fuel Cell Types & Working PEMFC, SOFC, MCFC, PAFC, AFC Fuel Cell What Are Fuel Cells Give Example fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or. What Are Fuel Cells Give Example.
From siqens.de
Principle, types, advantages What is a fuel cell? SIQENS What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into. What Are Fuel Cells Give Example.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind What Are Fuel Cells Give Example fuel cells work like batteries, but they do not run down or need recharging. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are combined to generate. They produce electricity and heat as long as fuel is supplied. . What Are Fuel Cells Give Example.
From www.nfcrc.uci.edu
National Fuel Cell Research Center (NFCRC), UC Irvine What Are Fuel Cells Give Example fuel cells work like batteries, but they do not run down or need recharging. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. a fuel cell uses the. What Are Fuel Cells Give Example.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies What Are Fuel Cells Give Example In short, they convert the chemical energy of fuels,. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel cells work like batteries, but. What Are Fuel Cells Give Example.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. In short, they convert the chemical energy of fuels,. In a fuel cell, hydrogen and oxygen are combined. What Are Fuel Cells Give Example.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The What Are Fuel Cells Give Example fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell is a type of galvanic cell that uses traditional. What Are Fuel Cells Give Example.
From lylagokejames.blogspot.com
Describe How a Fuel Cell Works What Are Fuel Cells Give Example In short, they convert the chemical energy of fuels,. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are combined to generate. They produce electricity and heat as long as fuel is supplied. a fuel cell uses the chemical. What Are Fuel Cells Give Example.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink What Are Fuel Cells Give Example a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. They produce electricity and heat as long as fuel is supplied. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In a fuel cell, hydrogen and oxygen are. What Are Fuel Cells Give Example.
From www.comsol.com
4 Examples of Fuel Cell Modeling in COMSOL Multiphysics® COMSOL Blog What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. In short, they convert the chemical energy of fuels,. a fuel cell is a device. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Fuel Cells PowerPoint Presentation, free download ID6040439 What Are Fuel Cells Give Example In a fuel cell, hydrogen and oxygen are combined to generate. In short, they convert the chemical energy of fuels,. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. They produce electricity and heat as long as fuel is supplied. a fuel cell is a type. What Are Fuel Cells Give Example.
From chem.libretexts.org
Case Study Fuel Cells Chemistry LibreTexts What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel. What Are Fuel Cells Give Example.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. fuel cells work like batteries, but they do not run. What Are Fuel Cells Give Example.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the What Are Fuel Cells Give Example In short, they convert the chemical energy of fuels,. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly. What Are Fuel Cells Give Example.
From www.chfca.ca
About Fuel Cells CHFCA What Are Fuel Cells Give Example fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert. What Are Fuel Cells Give Example.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell What Are Fuel Cells Give Example fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. fuel cells are devices that generate electricity through electrochemical redox reactions, not. What Are Fuel Cells Give Example.
From www.engineeringa2z.com
Fuel Cell Construction and Working Engineeringa2z What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. They produce electricity and heat as long as fuel is supplied. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells. What Are Fuel Cells Give Example.
From greenecon.net
Hydrogen Fuel Cells energy conversion and storage green econometrics What Are Fuel Cells Give Example fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. They produce electricity and heat as long as fuel is supplied. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is. What Are Fuel Cells Give Example.
From semiengineering.com
Fuel Cells And The IoE What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. In short, they convert the chemical energy of fuels,. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. a. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Types of Fuel Cells PowerPoint Presentation, free download ID What Are Fuel Cells Give Example In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells work like batteries, but they do not run down or need recharging. In short, they convert the chemical energy of fuels,. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into.. What Are Fuel Cells Give Example.
From www.alamy.com
How fuel cells work to produce electricity from hydrogen and air Stock What Are Fuel Cells Give Example a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. They produce electricity and heat as long as fuel is supplied. fuel cells work like batteries, but they do not run down or need recharging. In short, they convert the chemical energy of fuels,.. What Are Fuel Cells Give Example.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk What Are Fuel Cells Give Example fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. a fuel cell uses the chemical energy of hydrogen. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Fuel Cell Overview PowerPoint Presentation, free download ID What Are Fuel Cells Give Example a fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. They produce electricity and heat as long as fuel is supplied. fuel cells. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Fuel Cell Overview PowerPoint Presentation, free download ID What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. fuel cells are devices that generate electricity through electrochemical redox reactions, not combustion. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or. What Are Fuel Cells Give Example.
From www.researchgate.net
Types and applications of fuel cells [1]. Download Scientific Diagram What Are Fuel Cells Give Example fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or need recharging. In a fuel cell, hydrogen and oxygen are combined to generate. They produce electricity and heat as long as fuel is supplied. . What Are Fuel Cells Give Example.
From www.slideshare.net
Fuel cells and its types What Are Fuel Cells Give Example a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. In a fuel cell, hydrogen and oxygen are combined to generate. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells work like. What Are Fuel Cells Give Example.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working What Are Fuel Cells Give Example They produce electricity and heat as long as fuel is supplied. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In short, they convert the chemical energy of fuels,. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells work like batteries, but. What Are Fuel Cells Give Example.
From www.slideserve.com
PPT Hydrogen Fuel Cell Technology PowerPoint Presentation, free What Are Fuel Cells Give Example a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into. In a fuel cell, hydrogen and oxygen are combined to generate. fuel cells work like. What Are Fuel Cells Give Example.