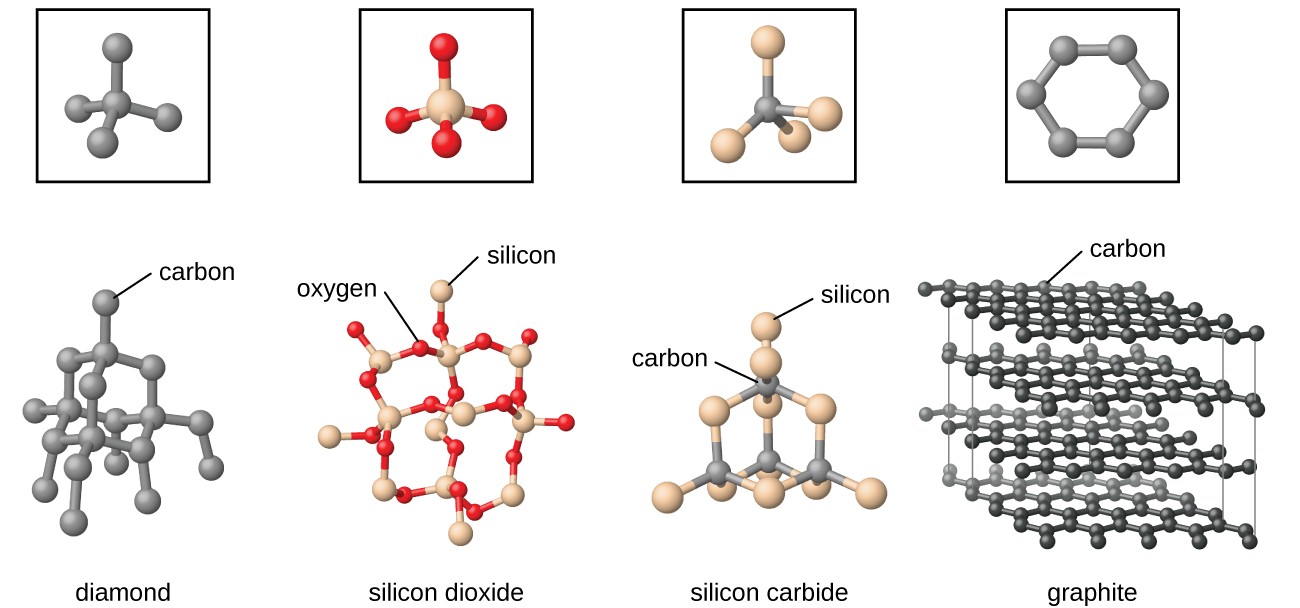
Is Graphite A Compound . It is not a compound, but a pure element with various types and uses. It is the most commonly used type and has a carbon content between 85 and 98 percent. It is not a compound, but a pure. Graphite is a native element mineral with the same composition as diamond, but with different properties. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Natural graphite is a crystalline form of carbon that is known as plumbago. Vein graphite is the rarest form and is likely. It is a soft, slippery, and cleavable material that forms by metamorphism. The flake form of natural graphite is used in refractory ceramics,. Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. Learn more about graphite structure, types, properties, and applications.
from www.askiitians.com
Learn more about graphite structure, types, properties, and applications. Natural graphite is a crystalline form of carbon that is known as plumbago. It is not a compound, but a pure element with various types and uses. Vein graphite is the rarest form and is likely. The flake form of natural graphite is used in refractory ceramics,. Graphite is a native element mineral with the same composition as diamond, but with different properties. It is not a compound, but a pure. It is the most commonly used type and has a carbon content between 85 and 98 percent. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is a soft, slippery, and cleavable material that forms by metamorphism.
Classification of Crystalline Solids Study Material for IIT JEE askIITians
Is Graphite A Compound Learn more about graphite structure, types, properties, and applications. Learn more about graphite structure, types, properties, and applications. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is not a compound, but a pure. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. Graphite is a native element mineral with the same composition as diamond, but with different properties. The flake form of natural graphite is used in refractory ceramics,. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Natural graphite is a crystalline form of carbon that is known as plumbago. Vein graphite is the rarest form and is likely. It is not a compound, but a pure element with various types and uses. It is the most commonly used type and has a carbon content between 85 and 98 percent. It is a soft, slippery, and cleavable material that forms by metamorphism.
From www.pinterest.com
Covalent Structure Of Graphite Graphite, Molecules, Molecular Is Graphite A Compound Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. The flake form of natural graphite is used in refractory ceramics,. It is the most commonly used type and has a carbon content between 85 and 98 percent. Graphite is a native element mineral with the. Is Graphite A Compound.
From fineartamerica.com
Graphite Molecular Structure Photograph by Mikkel Juul Jensen/science Photo Library Fine Art Is Graphite A Compound Natural graphite is a crystalline form of carbon that is known as plumbago. Vein graphite is the rarest form and is likely. It is not a compound, but a pure. The flake form of natural graphite is used in refractory ceramics,. It is a soft, slippery, and cleavable material that forms by metamorphism. It is the most commonly used type. Is Graphite A Compound.
From byjus.com
Describe the structure of Graphite. Is Graphite A Compound The flake form of natural graphite is used in refractory ceramics,. Graphite is a native element mineral with the same composition as diamond, but with different properties. It is a soft, slippery, and cleavable material that forms by metamorphism. It is the most commonly used type and has a carbon content between 85 and 98 percent. Vein graphite is the. Is Graphite A Compound.
From www.researchgate.net
The SEM images of the metallic compound graphite substrate (a) after... Download Scientific Is Graphite A Compound It is not a compound, but a pure. Natural graphite is a crystalline form of carbon that is known as plumbago. It is a soft, slippery, and cleavable material that forms by metamorphism. Learn more about graphite structure, types, properties, and applications. The flake form of natural graphite is used in refractory ceramics,. Graphite is a native element mineral with. Is Graphite A Compound.
From byjus.com
Graphite Structure & Uses Density & Crystal Chemistry Byju’s Is Graphite A Compound It is a soft, slippery, and cleavable material that forms by metamorphism. Vein graphite is the rarest form and is likely. Graphite is a native element mineral with the same composition as diamond, but with different properties. The flake form of natural graphite is used in refractory ceramics,. Graphite, on the other hand, is a crystalline form of carbon that. Is Graphite A Compound.
From www.researchgate.net
(a) SEM images of graphite (b) Graphite intercalation compound prepared... Download Scientific Is Graphite A Compound It is not a compound, but a pure. Learn more about graphite structure, types, properties, and applications. It is a soft, slippery, and cleavable material that forms by metamorphism. Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite is a native element mineral with the same composition as diamond, but with different properties. Graphite is. Is Graphite A Compound.
From www.researchgate.net
Schematic illustration of the chemical structure of graphite, graphene,... Download Scientific Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. The flake form of natural graphite is used in refractory ceramics,. Attractions between solvent molecules and carbon atoms will never be strong enough to. Natural graphite is a crystalline form of carbon that is known as plumbago. Vein graphite is the rarest form and. Is Graphite A Compound.
From mammothmemory.net
Examples of substances that have giant covalent bonds Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. The flake form of natural graphite is used in refractory ceramics,. Natural graphite is a crystalline form of carbon that. Is Graphite A Compound.
From www.dreamstime.com
The structure of graphite stock illustration. Illustration of atom 55335133 Is Graphite A Compound Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. It is a soft, slippery, and cleavable material that forms by metamorphism. Graphite is a native element mineral with the same composition as diamond, but with different properties. Learn more about graphite. Is Graphite A Compound.
From www.askiitians.com
Classification of Crystalline Solids Study Material for IIT JEE askIITians Is Graphite A Compound It is not a compound, but a pure element with various types and uses. Vein graphite is the rarest form and is likely. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. Learn more about graphite structure, types, properties, and applications. Graphite, on the other hand, is a crystalline form of carbon that is. Is Graphite A Compound.
From www.youtube.com
Structure of Graphite YouTube Is Graphite A Compound Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. It is a soft, slippery, and cleavable material that forms by metamorphism. Graphite is a native element mineral with the same composition as diamond, but with different properties. The flake form of natural graphite is used in refractory ceramics,. Learn more about graphite structure, types,. Is Graphite A Compound.
From www.savemyexams.com
Graphite structure AQA GCSE Chemistry Revision Notes 2018 Is Graphite A Compound The flake form of natural graphite is used in refractory ceramics,. It is a soft, slippery, and cleavable material that forms by metamorphism. It is the most commonly used type and has a carbon content between 85 and 98 percent. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is not a compound, but a. Is Graphite A Compound.
From socratic.org
What is the chemical formula of graphite? Socratic Is Graphite A Compound The flake form of natural graphite is used in refractory ceramics,. It is not a compound, but a pure element with various types and uses. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Natural graphite is a crystalline form of carbon that is known. Is Graphite A Compound.
From glossary.periodni.com
Graphite Chemistry Dictionary & Glossary Is Graphite A Compound Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. It is the most commonly used type and has a carbon content between 85 and 98 percent. Vein graphite is the rarest form and is likely. It is not a compound, but a pure. Graphite is. Is Graphite A Compound.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Is Graphite A Compound Natural graphite is a crystalline form of carbon that is known as plumbago. Vein graphite is the rarest form and is likely. It is a soft, slippery, and cleavable material that forms by metamorphism. It is the most commonly used type and has a carbon content between 85 and 98 percent. Attractions between solvent molecules and carbon atoms will never. Is Graphite A Compound.
From www.alamy.com
Graphite layers, threedimensional schematic diagram. Crystalline form of carbon atoms Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. The flake form of natural graphite is used in refractory ceramics,. It is a soft, slippery, and cleavable material that forms by metamorphism. Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite, on the other hand, is a crystalline. Is Graphite A Compound.
From alchetron.com
Graphite intercalation compound Alchetron, the free social encyclopedia Is Graphite A Compound It is the most commonly used type and has a carbon content between 85 and 98 percent. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. It is not a compound, but a pure. Vein graphite is the rarest form and is likely. Attractions between. Is Graphite A Compound.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Is Graphite A Compound It is not a compound, but a pure element with various types and uses. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Natural graphite is a crystalline form of. Is Graphite A Compound.
From www.researchgate.net
Graphite intercalation compounds and [15] Download Scientific Diagram Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. It is a soft, slippery, and cleavable material that forms by metamorphism. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. The flake form of natural graphite is used. Is Graphite A Compound.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Is Graphite A Compound Learn more about graphite structure, types, properties, and applications. Vein graphite is the rarest form and is likely. Graphite is a native element mineral with the same composition as diamond, but with different properties. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. The flake form of natural graphite is used in refractory ceramics,.. Is Graphite A Compound.
From www.dreamstime.com
Model of Graphite Molecular Structure Stock Image Image of chemistry, mineral 29809701 Is Graphite A Compound It is a soft, slippery, and cleavable material that forms by metamorphism. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is the most commonly used type and has a carbon content between 85 and 98 percent. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon. Is Graphite A Compound.
From www.animalia-life.club
Graphite Atom Structure Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. Learn more about graphite structure, types, properties, and applications. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. It is the most commonly used type and has a carbon. Is Graphite A Compound.
From www.youtube.com
STRUCTURE, PROPERTIES & USES OF GRAPHITE YouTube Is Graphite A Compound Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Graphite is a native element mineral with the same composition as diamond, but with different properties. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is not a compound, but a. Is Graphite A Compound.
From parcoscientific.com
Graphite Molecular Model Molecular Models Chemistry Is Graphite A Compound Natural graphite is a crystalline form of carbon that is known as plumbago. The flake form of natural graphite is used in refractory ceramics,. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. It is a soft, slippery, and cleavable material that forms by metamorphism. Graphite, on the other hand, is a crystalline form. Is Graphite A Compound.
From mineralseducationcoalition.org
Graphite Minerals Education Coalition Is Graphite A Compound Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Graphite is a native element mineral with the same composition as diamond, but with different properties. Learn more about graphite structure, types, properties, and applications. It is not a compound, but a pure. Vein graphite is. Is Graphite A Compound.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Is Graphite A Compound Vein graphite is the rarest form and is likely. It is not a compound, but a pure element with various types and uses. Learn more about graphite structure, types, properties, and applications. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. It is not a compound, but a pure. It is a soft, slippery,. Is Graphite A Compound.
From animalia-life.club
Structure Of Graphite Model Is Graphite A Compound Learn more about graphite structure, types, properties, and applications. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Vein graphite is the rarest form and is likely. It is the. Is Graphite A Compound.
From pressbooks.bccampus.ca
5.2 Bonding and Lattices Physical Geology H5P Edition V1.1 Is Graphite A Compound Vein graphite is the rarest form and is likely. Natural graphite is a crystalline form of carbon that is known as plumbago. The flake form of natural graphite is used in refractory ceramics,. Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite, on the other hand, is a crystalline form of carbon that is composed. Is Graphite A Compound.
From www.slideserve.com
PPT Graphite intercalation compounds PowerPoint Presentation, free download ID1989896 Is Graphite A Compound Graphite is a native element mineral with the same composition as diamond, but with different properties. It is not a compound, but a pure. The flake form of natural graphite is used in refractory ceramics,. Natural graphite is a crystalline form of carbon that is known as plumbago. Graphite is a form of carbon with a hexagonal lattice of carbon. Is Graphite A Compound.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID2654690 Is Graphite A Compound It is not a compound, but a pure. Learn more about graphite structure, types, properties, and applications. Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon atoms arranged in a hexagonal lattice. Vein graphite is the rarest form. Is Graphite A Compound.
From www.savemyexams.com
Giant Covalent Structures SL IB Chemistry Revision Notes 2025 Save My Exams Is Graphite A Compound Attractions between solvent molecules and carbon atoms will never be strong enough to. Learn more about graphite structure, types, properties, and applications. The flake form of natural graphite is used in refractory ceramics,. Graphite is a native element mineral with the same composition as diamond, but with different properties. It is a soft, slippery, and cleavable material that forms by. Is Graphite A Compound.
From geologyscience.com
Graphite Mineral Physical Optical Properties, Uses, Occurrence Is Graphite A Compound Learn more about graphite structure, types, properties, and applications. It is not a compound, but a pure. It is a soft, slippery, and cleavable material that forms by metamorphism. Graphite is a native element mineral with the same composition as diamond, but with different properties. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers.. Is Graphite A Compound.
From www.slideserve.com
PPT Graphite intercalation compounds PowerPoint Presentation, free download ID1989896 Is Graphite A Compound Attractions between solvent molecules and carbon atoms will never be strong enough to. Graphite is a native element mineral with the same composition as diamond, but with different properties. Natural graphite is a crystalline form of carbon that is known as plumbago. Graphite, on the other hand, is a crystalline form of carbon that is composed of layers of carbon. Is Graphite A Compound.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Is Graphite A Compound Natural graphite is a crystalline form of carbon that is known as plumbago. Graphite is a form of carbon with a hexagonal lattice of carbon atoms in layers. It is the most commonly used type and has a carbon content between 85 and 98 percent. Attractions between solvent molecules and carbon atoms will never be strong enough to. It is. Is Graphite A Compound.
From mavink.com
Diagram Of Graphite Structure Is Graphite A Compound Attractions between solvent molecules and carbon atoms will never be strong enough to. Learn more about graphite structure, types, properties, and applications. Natural graphite is a crystalline form of carbon that is known as plumbago. It is the most commonly used type and has a carbon content between 85 and 98 percent. It is not a compound, but a pure.. Is Graphite A Compound.