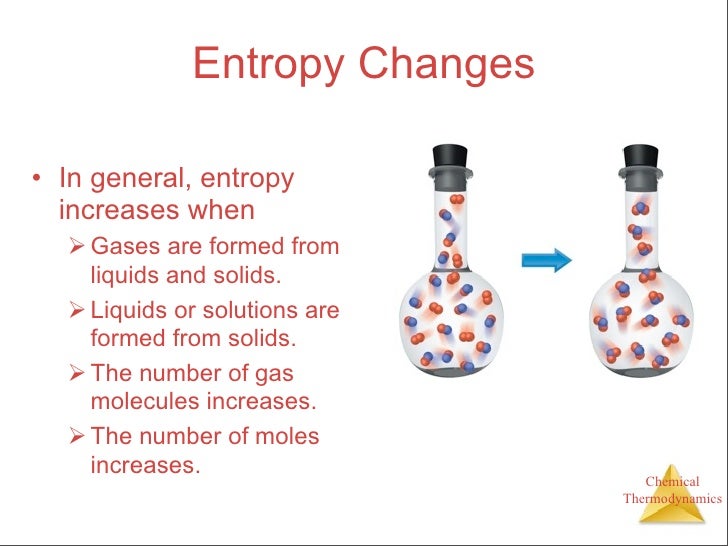
When Solid Changes Into Liquid The Entropy Sign Is Negative . For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The entropy of the system, which is δq/t, increases by δq/273k. The heat δq for this process is the energy required to change water. The liquid becomes a more ordered solid. Entropy changes during physical changes. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. This includes solid to liquid, liquid to gas and solid to aqueous solution. By the same logic, the reciprocal. The solid dissolves to give an increase of mobile ions in solution. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures.
from www.slideshare.net
As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The heat δq for this process is the energy required to change water. The liquid becomes a more ordered solid. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. Entropy changes during physical changes. The solid dissolves to give an increase of mobile ions in solution. By the same logic, the reciprocal. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in.
Chapter 19 Lecture Thermodynamics
When Solid Changes Into Liquid The Entropy Sign Is Negative The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. By the same logic, the reciprocal. The solid dissolves to give an increase of mobile ions in solution. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. This includes solid to liquid, liquid to gas and solid to aqueous solution. The heat δq for this process is the energy required to change water. The entropy of the system, which is δq/t, increases by δq/273k. Entropy changes during physical changes. The liquid becomes a more ordered solid. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or.
From byjus.com
The entropy change associated with the conversion of 1 kg of ice at 2 vapours at 383 K is When Solid Changes Into Liquid The Entropy Sign Is Negative The heat δq for this process is the energy required to change water. The entropy of the system, which is δq/t, increases by δq/273k. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. As a result, s liquid > s. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Entropy and Free Energy PowerPoint Presentation, free download ID3943123 When Solid Changes Into Liquid The Entropy Sign Is Negative The heat δq for this process is the energy required to change water. The liquid becomes a more ordered solid. By the same logic, the reciprocal. Entropy changes during physical changes. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. The entropy of. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From courses.lumenlearning.com
Entropy Chemistry Atoms First When Solid Changes Into Liquid The Entropy Sign Is Negative As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. This includes solid to liquid, liquid to gas and solid to aqueous solution. As a result, s liquid > s solid and the process of converting a substance from solid. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From easyscienceforkids.com
Changes in Matter Phases States of Matter Image When Solid Changes Into Liquid The Entropy Sign Is Negative The solid dissolves to give an increase of mobile ions in solution. The liquid becomes a more ordered solid. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. The heat δq for this process is the energy required to change water. The decrease. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.youtube.com
⚗️ Predicting the Sign of Entropy Change YouTube When Solid Changes Into Liquid The Entropy Sign Is Negative The solid dissolves to give an increase of mobile ions in solution. The entropy of the system, which is δq/t, increases by δq/273k. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Evaluating entropy changes PowerPoint Presentation, free download ID2088185 When Solid Changes Into Liquid The Entropy Sign Is Negative Entropy changes during physical changes. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. For a given. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 7 ENTROPY PowerPoint Presentation, free download ID8672927 When Solid Changes Into Liquid The Entropy Sign Is Negative As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. The liquid becomes a more ordered solid. By the same logic, the reciprocal. The heat δq for this process is the energy required to change water. This includes solid to. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From study.com
Entropy in Chemistry Definition & Calculation Lesson When Solid Changes Into Liquid The Entropy Sign Is Negative Entropy changes during physical changes. The heat δq for this process is the energy required to change water. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative,. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID2799024 When Solid Changes Into Liquid The Entropy Sign Is Negative The heat δq for this process is the energy required to change water. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. Entropy changes during physical changes. By the same logic, the reciprocal. When both the change in entropy of. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID2017581 When Solid Changes Into Liquid The Entropy Sign Is Negative The heat δq for this process is the energy required to change water. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. By the same logic, the reciprocal. The entropy of the system, which is δq/t, increases by δq/273k.. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From general.chemistrysteps.com
Entropy Changes in the Surroundings Chemistry Steps When Solid Changes Into Liquid The Entropy Sign Is Negative For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The heat δq for this process is the energy required to change water. The solid dissolves to give an increase of mobile ions in solution. Entropy changes during physical changes. The. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 8, pt 4 of 5 Entropy of Liquids and Solids YouTube When Solid Changes Into Liquid The Entropy Sign Is Negative When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. The entropy of the system, which is δq/t,. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From dynamichop.weebly.com
How to calculate entropy change thermodynamics dynamicHop When Solid Changes Into Liquid The Entropy Sign Is Negative The solid dissolves to give an increase of mobile ions in solution. The heat δq for this process is the energy required to change water. By the same logic, the reciprocal. The liquid becomes a more ordered solid. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.thesciencehive.co.uk
Enthalpy and Entropy* — the science sauce When Solid Changes Into Liquid The Entropy Sign Is Negative The entropy of the system, which is δq/t, increases by δq/273k. The heat δq for this process is the energy required to change water. Entropy changes during physical changes. The solid dissolves to give an increase of mobile ions in solution. This includes solid to liquid, liquid to gas and solid to aqueous solution. As a result, s liquid >. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.youtube.com
How To Calculate Entropy Changes Liquids, Solids, and Phase Changes YouTube When Solid Changes Into Liquid The Entropy Sign Is Negative By the same logic, the reciprocal. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT What is Entropy? Tds Relations and Δ S of Liquids and Solids PowerPoint Presentation ID When Solid Changes Into Liquid The Entropy Sign Is Negative Entropy changes during physical changes. This includes solid to liquid, liquid to gas and solid to aqueous solution. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. For a given substance, s solid < s liquid < s gas in a given physical state at. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From jackwestin.com
Second Law Concept Of Entropy Energy Changes In Chemical Reactions MCAT Content When Solid Changes Into Liquid The Entropy Sign Is Negative By the same logic, the reciprocal. The heat δq for this process is the energy required to change water. The liquid becomes a more ordered solid. This includes solid to liquid, liquid to gas and solid to aqueous solution. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 16 PowerPoint Presentation, free download ID5604541 When Solid Changes Into Liquid The Entropy Sign Is Negative As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. The liquid becomes a more ordered solid. As. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From mungfali.com
Ppt Chapter 19 Spontaneous Change Entropy And Free Energy 3B5 When Solid Changes Into Liquid The Entropy Sign Is Negative The liquid becomes a more ordered solid. The heat δq for this process is the energy required to change water. The solid dissolves to give an increase of mobile ions in solution. The entropy of the system, which is δq/t, increases by δq/273k. As a result, s liquid > s solid and the process of converting a substance from solid. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 18 Entropy, Free Energy, and Equilibrium Part I PowerPoint Presentation ID6769763 When Solid Changes Into Liquid The Entropy Sign Is Negative Entropy changes during physical changes. The solid dissolves to give an increase of mobile ions in solution. The liquid becomes a more ordered solid. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. For a given substance, s solid < s liquid < s gas. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps When Solid Changes Into Liquid The Entropy Sign Is Negative By the same logic, the reciprocal. Entropy changes during physical changes. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The entropy of the system, which is δq/t, increases by δq/273k. As a result, s liquid > s solid and the process of converting a. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 19 Entropy and Free Energy PowerPoint Presentation, free download ID3580964 When Solid Changes Into Liquid The Entropy Sign Is Negative As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in entropy, δs > 0. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. When both the change in entropy. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.snexplores.org
Explainer What are the different states of matter? When Solid Changes Into Liquid The Entropy Sign Is Negative The liquid becomes a more ordered solid. The solid dissolves to give an increase of mobile ions in solution. The heat δq for this process is the energy required to change water. This includes solid to liquid, liquid to gas and solid to aqueous solution. As a result, s liquid > s solid and the process of converting a substance. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 18 Entropy, Free Energy, and Equilibrium PowerPoint Presentation ID5918904 When Solid Changes Into Liquid The Entropy Sign Is Negative When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. By the same logic, the reciprocal. The solid dissolves to give an increase of mobile ions in solution. The entropy of the system, which is δq/t, increases by δq/273k. The decrease in moles of. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideshare.net
Chapter 19 Lecture Thermodynamics When Solid Changes Into Liquid The Entropy Sign Is Negative The solid dissolves to give an increase of mobile ions in solution. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Chapter 19 Thermodynamics PowerPoint Presentation, free download ID6038928 When Solid Changes Into Liquid The Entropy Sign Is Negative By the same logic, the reciprocal. The entropy of the system, which is δq/t, increases by δq/273k. The liquid becomes a more ordered solid. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. As a result, s liquid > s. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From scienceinfo.com
Entropy Definition, Properties, and Facts When Solid Changes Into Liquid The Entropy Sign Is Negative The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The solid dissolves to give an increase of mobile ions in solution. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From interestingengineering.com
An Infinite Disorder The Physics of Entropy When Solid Changes Into Liquid The Entropy Sign Is Negative This includes solid to liquid, liquid to gas and solid to aqueous solution. By the same logic, the reciprocal. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. The liquid becomes a more ordered solid. The heat δq for this process is the. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.youtube.com
Thermodynamics Total Entropy Change Calculation Solid/Liquid YouTube When Solid Changes Into Liquid The Entropy Sign Is Negative This includes solid to liquid, liquid to gas and solid to aqueous solution. By the same logic, the reciprocal. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process of converting a substance. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.grc.nasa.gov
Entropy of a Gas When Solid Changes Into Liquid The Entropy Sign Is Negative When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The heat δq for this process is the energy required. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideserve.com
PPT Entropy Randomness & Disorder PowerPoint Presentation ID6039529 When Solid Changes Into Liquid The Entropy Sign Is Negative The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The heat δq for this process is the energy required to change water. The solid dissolves to give an increase of mobile ions in solution. When both the change in entropy of the system and the. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From sciencenotes.org
What Is Entropy? Definition and Examples When Solid Changes Into Liquid The Entropy Sign Is Negative This includes solid to liquid, liquid to gas and solid to aqueous solution. The decrease in moles of gas in the haber ammonia synthesis drives the entropy change negative, making the reaction spontaneous only at low temperatures. The liquid becomes a more ordered solid. The solid dissolves to give an increase of mobile ions in solution. The entropy of the. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From opentextbc.ca
Measuring Entropy and Entropy Changes Introductory Chemistry 1st Canadian Edition When Solid Changes Into Liquid The Entropy Sign Is Negative The solid dissolves to give an increase of mobile ions in solution. This includes solid to liquid, liquid to gas and solid to aqueous solution. As a result, s liquid > s solid and the process of converting a substance from solid to liquid (melting) is characterized by an increase in. By the same logic, the reciprocal. The decrease in. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers When Solid Changes Into Liquid The Entropy Sign Is Negative Entropy changes during physical changes. The liquid becomes a more ordered solid. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. As a result, s liquid > s solid and the process of converting a substance from solid to liquid. When Solid Changes Into Liquid The Entropy Sign Is Negative.
From www.slideshare.net
10.3 Entropy and the 2nd law When Solid Changes Into Liquid The Entropy Sign Is Negative The liquid becomes a more ordered solid. This includes solid to liquid, liquid to gas and solid to aqueous solution. When both the change in entropy of the system and the change in entropy of the surroundings are positive, the change in entropy of the universe. As a result, s liquid > s solid and the process of converting a. When Solid Changes Into Liquid The Entropy Sign Is Negative.