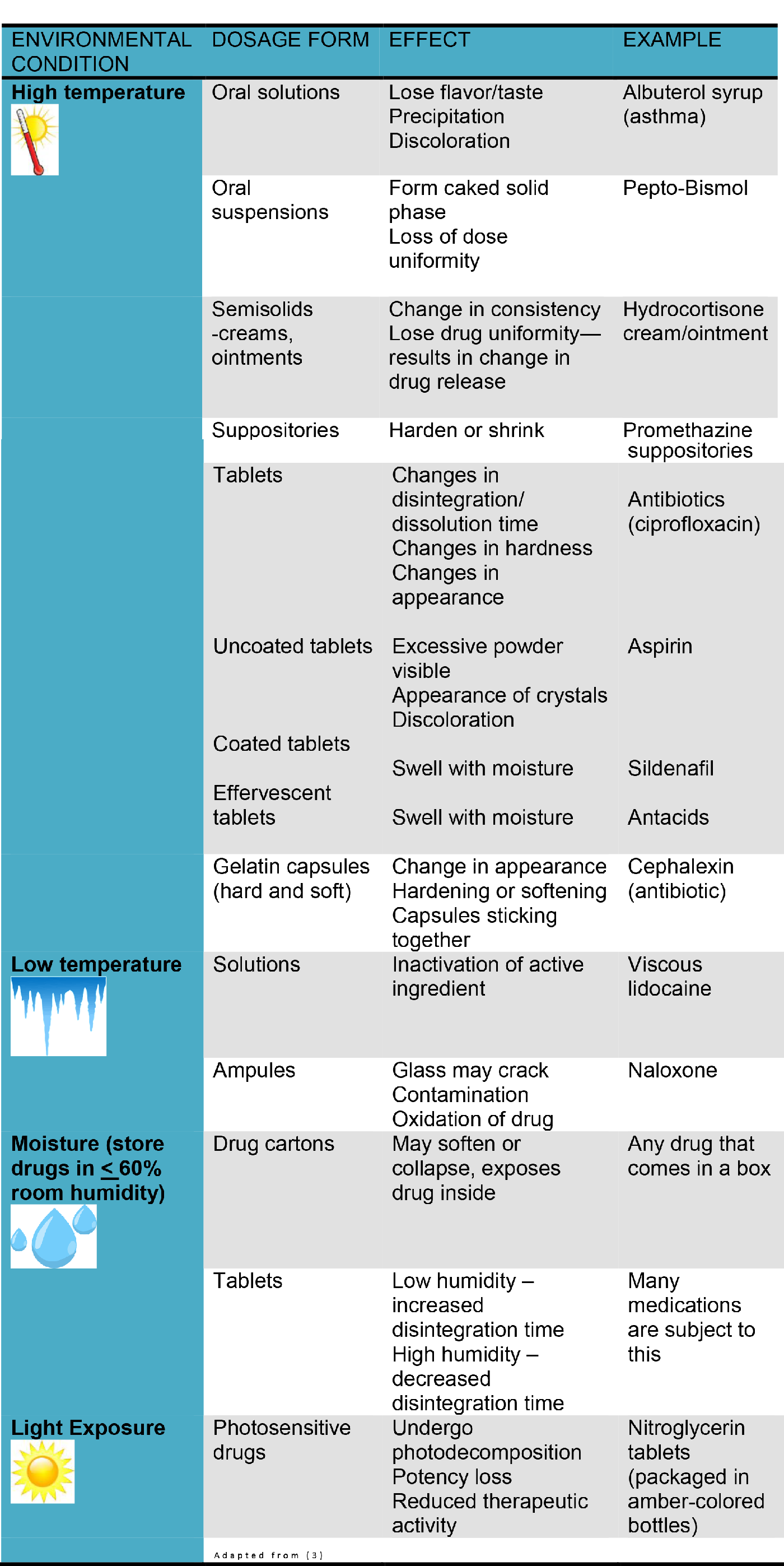
Drug Storage Temperature Guidelines . usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. good storage and distribution practices for medical products. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for environmental control of drugs during storage and transportation : guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight.
from www.emergency-live.com
guidelines for environmental control of drugs during storage and transportation : good storage and distribution practices for medical products. guidelines for temperature control of drug products during storage and transportation (gui0069). temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight.
How to store medicines in wilderness and EMS environment Read the guidelines!
Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for temperature control of drug products during storage and transportation (gui0069). temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. good storage and distribution practices for medical products. guidelines for environmental control of drugs during storage and transportation : usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with.
From sophiemetcalfe.z13.web.core.windows.net
Temperature Controlled Storage For Medication Drug Storage Temperature Guidelines good storage and distribution practices for medical products. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). guidelines for environmental control of drugs during storage and transportation : temperature profiling for. Drug Storage Temperature Guidelines.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for environmental control of drugs during storage and transportation : guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons (individuals and companies) who store and/or. Drug Storage Temperature Guidelines.
From www.accuweather.com
Heat and medication Pharmacists share tips to keep your prescriptions safe Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from. Drug Storage Temperature Guidelines.
From www.researchgate.net
Compliance to drug storage temperature criteria for oral anticancer... Download Table Drug Storage Temperature Guidelines good storage and distribution practices for medical products. guidelines for environmental control of drugs during storage and transportation : usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry. Drug Storage Temperature Guidelines.
From classlibrarymoses.z4.web.core.windows.net
Covid Vaccine Storage Temperature Chart Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for environmental control of drugs during storage and transportation : guidelines for temperature control of drug products. Drug Storage Temperature Guidelines.
From sentrybps.com
Controlled Room Temperature Enhances Your Supply Chain Drug Storage Temperature Guidelines good storage and distribution practices for medical products. guidelines for temperature control of drug products during storage and transportation (gui0069). in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing. Drug Storage Temperature Guidelines.
From trumedsystems.com
COVID19 Vaccine Temperature Stability Chart TruMed Systems Drug Storage Temperature Guidelines good storage and distribution practices for medical products. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. in general, most medicines should be stored at 59 to 77 degrees f in. Drug Storage Temperature Guidelines.
From www.jem-journal.com
Drug storage temperatures in rescue vehicles1 Journal of Emergency Medicine Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. guidelines for environmental control of drugs during storage and transportation : these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. good storage and distribution practices for medical products.. Drug Storage Temperature Guidelines.
From www.sensoscientific.com
Pharmaceutical Temperature Mapping Why Is It Needed? SensoScientific Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for environmental control of drugs during storage and transportation : good storage and distribution practices for medical products. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. guidelines for. Drug Storage Temperature Guidelines.
From wms.org
TemperatureStability Drug Storage Temperature Guidelines good storage and distribution practices for medical products. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons (individuals and companies) who store and/or transport drugs to. Drug Storage Temperature Guidelines.
From www.crspharmasolutions.ie
Pharmaceutical Temperature Ranges CRS Pharma Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. in general,. Drug Storage Temperature Guidelines.
From q1scientific.com
A Q&A guide to stability storage Q1 Scientific Drug Storage Temperature Guidelines guidelines for temperature control of drug products during storage and transportation (gui0069). temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored. Drug Storage Temperature Guidelines.
From www.akcp.com
Temperature Excursion Management in Pharmaceutical Storage Drug Storage Temperature Guidelines these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. usp. Drug Storage Temperature Guidelines.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Drug Storage Temperature Guidelines these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. guidelines for temperature control of drug products during storage and transportation (gui0069). guidelines for environmental control of drugs during storage and transportation : usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs. Drug Storage Temperature Guidelines.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. guidelines for environmental control of drugs during storage and transportation : good storage and distribution practices for medical. Drug Storage Temperature Guidelines.
From www.health.gov.au
National vaccine storage guidelines ‘Strive for 5’ Vaccine fridge temperature chart poster Drug Storage Temperature Guidelines guidelines for environmental control of drugs during storage and transportation : in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. good storage and distribution practices for medical products. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply. Drug Storage Temperature Guidelines.
From www.academia.edu
(PDF) Roomtemperature storage of medications labeled for refrigeration Lefteris Teperikidis Drug Storage Temperature Guidelines guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. good storage and distribution practices for medical products. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. in. Drug Storage Temperature Guidelines.
From prescriptionhope.com
Medication Storage, Where, How, Temperatures, Types, and Guidelines Drug Storage Temperature Guidelines good storage and distribution practices for medical products. guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the. Drug Storage Temperature Guidelines.
From q1scientific.com
ICH Quality Guidelines for Pharmaceutical Stability Storage Q1 Scientific Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for environmental control of drugs during storage and transportation : usp <<strong>1079</strong>> outlines seven key recommendations to. Drug Storage Temperature Guidelines.
From prescriptionhope.com
Medication Storage, Where, How, Temperatures, Types, and Guidelines Drug Storage Temperature Guidelines guidelines for environmental control of drugs during storage and transportation : these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. good storage and distribution practices for medical. Drug Storage Temperature Guidelines.
From jodiebarnes.z21.web.core.windows.net
Cdc Medication Storage Temperature Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. good storage and distribution practices for medical products. guidelines for temperature control of drug products during storage and transportation (gui0069). temperature profiling for warehouses already in use should be done at known times of external. Drug Storage Temperature Guidelines.
From www.akcp.com
Temperature Excursion Management in Pharmaceutical Storage Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. temperature profiling for warehouses already in use should be done at known times of external temperature. Drug Storage Temperature Guidelines.
From trumedsystems.com
Flu Vaccine Refrigeration 2022 TruMed Systems Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). good storage and distribution practices for medical products. temperature profiling for warehouses already in use should be done at known times of external. Drug Storage Temperature Guidelines.
From vaccines.phila.gov
Storage and Handling Philadelphia Immunization Program Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply with. guidelines for environmental control of drugs during storage and transportation : temperature profiling for warehouses already in use should. Drug Storage Temperature Guidelines.
From www.akcp.com
Monitoring Pharmaceutical Storage Temperature AKCP Solutions Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for temperature control of drug products during storage and transportation (gui0069). guidelines for environmental control of drugs. Drug Storage Temperature Guidelines.
From www.scribd.com
Guidelines for Maintaining Temperature Control of Drug Products During Storage and Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for environmental control of drugs during storage and transportation : guidelines for temperature control of drug products. Drug Storage Temperature Guidelines.
From alithadnews.com
Pharmaceutical Temperature Controlled Storage Alit Had News Get expert tips and advices Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from. Drug Storage Temperature Guidelines.
From dandkmotorsports.com
Botox Storage Requirements Dandk Organizer Drug Storage Temperature Guidelines in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. good storage and distribution practices for medical products. usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. these guidelines will help all persons (individuals. Drug Storage Temperature Guidelines.
From q1scientific.com
ICH Quality Guidelines for Pharmaceutical Stability Storage Q1 Scientific Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). these guidelines will help all persons. Drug Storage Temperature Guidelines.
From www.tempalert.com
Temperature is Critical for Safe, Effective Refrigerated Drug Storage Drug Storage Temperature Guidelines good storage and distribution practices for medical products. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing. Drug Storage Temperature Guidelines.
From www.emergency-live.com
How to store medicines in wilderness and EMS environment Read the guidelines! Drug Storage Temperature Guidelines temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. good storage and distribution practices for medical products. guidelines for environmental control of drugs during storage and transportation : in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from. Drug Storage Temperature Guidelines.
From blog.smartsense.co
Temperature is Critical for Safe, Effective Refrigerated Drug Storage Drug Storage Temperature Guidelines good storage and distribution practices for medical products. guidelines for temperature control of drug products during storage and transportation (gui0069). in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for environmental control of drugs during storage and transportation : temperature profiling for. Drug Storage Temperature Guidelines.
From www.scribd.com
Guidelines For Temperature Control of Drug Products During Storage and Transportation Gui0069 Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. in general, most medicines should be stored at 59 to 77 degrees f in a cool, dry place, away from sunlight. guidelines for temperature control of drug products during storage and transportation (gui0069). good storage and. Drug Storage Temperature Guidelines.
From www.emergency-live.com
How to store medicines in wilderness and EMS environment Read the guidelines! Drug Storage Temperature Guidelines usp <<strong>1079</strong>> outlines seven key recommendations to ensure your pharmaceutical storage units are always storing drugs safely within the manufacturer’s. good storage and distribution practices for medical products. guidelines for environmental control of drugs during storage and transportation : temperature profiling for warehouses already in use should be done at known times of external temperature extremes,.. Drug Storage Temperature Guidelines.
From present5.com
Storage and Record Keeping Requirements for Transfusable Blood Drug Storage Temperature Guidelines guidelines for temperature control of drug products during storage and transportation (gui0069). temperature profiling for warehouses already in use should be done at known times of external temperature extremes,. guidelines for environmental control of drugs during storage and transportation : these guidelines will help all persons (individuals and companies) who store and/or transport drugs to comply. Drug Storage Temperature Guidelines.