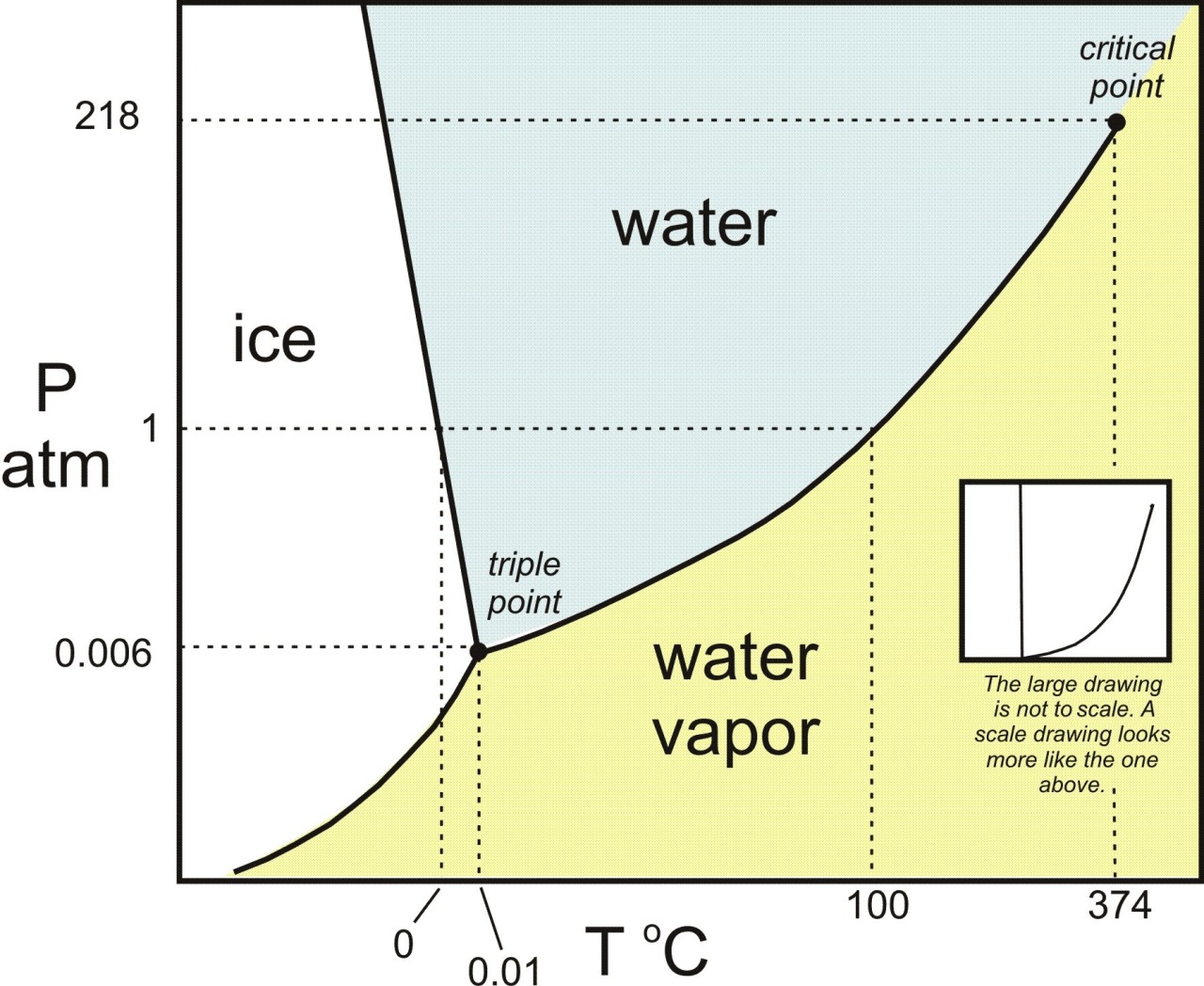
Give A Reason Why Solids And Liquids Coexist At Their Melting Point . It is unique to a substance and is dependent on the pressure. Take water (h 2 o) as an. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. This is the point at which both liquid and solid phase exists at. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The temperature at which solid and liquid states coexist together is called the melting point.
from invaderxan.tumblr.com
Take water (h 2 o) as an. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. It is unique to a substance and is dependent on the pressure. The temperature at which solid and liquid states coexist together is called the melting point. This is the point at which both liquid and solid phase exists at. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form.
Mostly Harmless — Phase diagram for water. The triple point is the...
Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Take water (h 2 o) as an. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. This is the point at which both liquid and solid phase exists at. The temperature at which solid and liquid states coexist together is called the melting point.
From susiedhardy.blob.core.windows.net
Solids Melting Point at susiedhardy blog Give A Reason Why Solids And Liquids Coexist At Their Melting Point For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. Take water (h 2 o) as an. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid changes its state to liquid at atmospheric pressure is called the. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.britannica.com
solid Definition & Facts Britannica Give A Reason Why Solids And Liquids Coexist At Their Melting Point First, at a substance’s melting point or boiling point, two phases can exist simultaneously. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid and liquid states coexist together is called the melting point. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. The. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid and liquid states coexist together is called the. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board, Class 7. Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The temperature at which solid and liquid states coexist together is called the melting point. It is unique to a substance and is dependent on the pressure. The melting point is the temperature where the solid and liquid phases. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
MATTER & ITS PROPERTIES NOTESHEET ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The melting point is the temperature where the solid and. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.coursehero.com
Solid to Gas Phase Transition Introduction to Chemistry Course Hero Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). It is unique to a substance and is dependent on the pressure. This is the point at which both liquid and solid phase exists at. The temperature at. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. This is the point at which both liquid and solid phase exists at. The temperature at which solid and liquid states coexist together is called the melting point. It is unique to a substance and is dependent on the pressure. For example, ice is a solid form of water that melts at 0. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.snexplores.org
Explainer What are the different states of matter? Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. It is unique to a substance and is dependent on the pressure. This is the point at. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID998654 Give A Reason Why Solids And Liquids Coexist At Their Melting Point For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. This is the point. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Solids and Liquids Chapter 14 Chem B. ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Take water (h 2 o) as an. The temperature at which solid and liquid states coexist together is called the melting point. First, at a substance’s melting. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Take water (h 2 o) as an. This is the point at which both liquid and solid phase exists at. The temperature at which solid and liquid. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT Chapter 10 Liquids, Solids, and Phase Change PowerPoint Presentation ID705987 Give A Reason Why Solids And Liquids Coexist At Their Melting Point First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. The temperature at which solid and liquid states coexist together is called the melting point. The melting point is the temperature where the solid and liquid phases. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams JoVE Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Take water (h 2 o) as an. This is the point at which both liquid and solid phase exists at. It is unique to a substance and is dependent on the pressure. The temperature at. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From dokumen.tips
(PDF) Unit 6 Solids, Liquids and Solutions Pages/NOTES/Solids, Liquids, and... · Unit 6 Give A Reason Why Solids And Liquids Coexist At Their Melting Point For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. The temperature at which solid and liquid states coexist together is called the melting point. This is the point at which both liquid and solid phase exists at. First, at a substance’s melting point or boiling point,. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Phase Changes. ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. For example, ice is a solid. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. The melting. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From sansona.github.io
Solids, Liquids, and Gases Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. This is the point at which. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in each of the three states of Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.ase.org.uk
Solids, liquids and gases Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. This is the point at which both liquid and solid phase exists at. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. First, at a substance’s melting point or boiling point, two phases can exist simultaneously.. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Chapter 16 States of Matter. ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. The temperature at which solid and liquid states coexist together is called the melting point. Take water (h 2 o) as an. This is the point at. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Phase Changes. ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point Take water (h 2 o) as an. The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From invaderxan.tumblr.com
Mostly Harmless — Phase diagram for water. The triple point is the... Give A Reason Why Solids And Liquids Coexist At Their Melting Point For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. This is the point at which both liquid and solid phase exists at. Take water (h 2 o) as an. It is unique. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Liquids and Solids Chapter ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature at which solid and liquid states coexist together is called the melting point. It is unique to a substance. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
States and Changes of Matter ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Take water (h 2 o) as an. The temperature at which solid changes its state to liquid at atmospheric pressure is called the. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (h 2 o) as an. This is the point at which both liquid and solid phase exists at. For example, ice is a solid form of water that melts at 0 degrees. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. For example, ice is a solid form of water. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Intermolecular Forces and ppt download Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Give A Reason Why Solids And Liquids Coexist At Their Melting Point This is the point at which both liquid and solid phase exists at. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). It is unique to a substance and is dependent on the pressure. Take water (h 2 o) as an. First, at a. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.primaryworks.co.uk
PowerPoint KS2 explanation on solids, liquids & gases KS2 primary science unit Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. Take water (h 2 o) as an. The temperature at which solid. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT Chapter 10 Liquids, Solids, and Phase Change PowerPoint Presentation ID705987 Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid and liquid states coexist together is called the melting point. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.vrogue.co
How Does Temperature Affect Solids Liquids And Gases vrogue.co Give A Reason Why Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure. This is the point at which both liquid and solid phase exists at. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. The temperature at which solid changes its state to liquid at atmospheric. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From slidetodoc.com
Solids Liquids and Intermolecular forces Objective 1 Learn Give A Reason Why Solids And Liquids Coexist At Their Melting Point First, at a substance’s melting point or boiling point, two phases can exist simultaneously. For example, ice is a solid form of water that melts at 0 degrees celsius/32 degrees fahrenheit and changes to its liquid form. This is the point at which both liquid and solid phase exists at. The temperature at which solid changes its state to liquid. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From mavink.com
Phases Of Matter Diagram Give A Reason Why Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Take water (h 2 o) as an. It is unique to a substance and is dependent on the pressure. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. The temperature. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT Solids and Liquids PowerPoint Presentation, free download ID2093811 Give A Reason Why Solids And Liquids Coexist At Their Melting Point The temperature at which solid and liquid states coexist together is called the melting point. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. This is the point at which both liquid and solid phase exists at. The melting point is the temperature where the solid and liquid. Give A Reason Why Solids And Liquids Coexist At Their Melting Point.