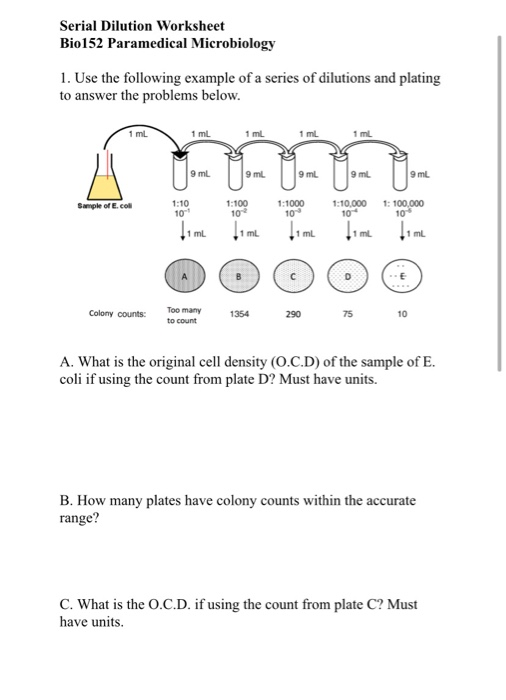
Molarity And Dilution Worksheet Answers Chemistry . Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Then use that as the initial concentration for the second. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity.
from lessondbjack.z13.web.core.windows.net
What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. For chemistry help, visit www.chemfiesta.com. Then use that as the initial concentration for the second. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. First find the concentration of solution a.
Dilutions Worksheet Chemistry Answers
Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. For chemistry help, visit www.chemfiesta.com. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. First find the concentration of solution a. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration for the second. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution.
From www.pinterest.com
Molarity and Dilutions Notes and Worksheet Set Teaching chemistry, Chemistry basics Molarity And Dilution Worksheet Answers Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Molarity review problems 1) what is the molarity of a solution. Molarity And Dilution Worksheet Answers Chemistry.
From davida.davivienda.com
Molarity And Dilution Worksheet Answers Chemistry Printable Word Searches Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Then use that as the initial concentration for the second. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of. Molarity And Dilution Worksheet Answers Chemistry.
From worksheets.clipart-library.com
Solved CHEM 101 Worksheet 11 molarity & dilution Name 1. Worksheets Library Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Molarity review. Molarity And Dilution Worksheet Answers Chemistry.
From davida.davivienda.com
Molarity And Dilution Worksheet Answers Chemistry Printable Word Searches Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. Then use that as the initial concentration for the second. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. For chemistry help, visit www.chemfiesta.com.. Molarity And Dilution Worksheet Answers Chemistry.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Molarity And Dilution Worksheet Answers Chemistry What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the. Molarity And Dilution Worksheet Answers Chemistry.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. For chemistry help, visit www.chemfiesta.com. What is the molarity of 2.0 l of solution made from 2.4. Molarity And Dilution Worksheet Answers Chemistry.
From davida.davivienda.com
Molarity And Dilution Worksheet Answer Key Printable Word Searches Molarity And Dilution Worksheet Answers Chemistry Then use that as the initial concentration for the second. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. For chemistry help, visit www.chemfiesta.com. Molarity review problems 1) what is the. Molarity And Dilution Worksheet Answers Chemistry.
From aznswerzonefoganklebones.z13.web.core.windows.net
Molarity Worksheet With Answers Molarity And Dilution Worksheet Answers Chemistry Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration for the second. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml. Molarity And Dilution Worksheet Answers Chemistry.
From lessonlibrarycarmen.z21.web.core.windows.net
Molarity By Dilution Worksheets Answers Molarity And Dilution Worksheet Answers Chemistry Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. 1) if i have 340 ml of a. Molarity And Dilution Worksheet Answers Chemistry.
From lessondbjack.z13.web.core.windows.net
Dilutions Worksheet Chemistry Answers Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Then use that as the initial concentration for the second. For chemistry help, visit www.chemfiesta.com. What is the molarity of 2.0 l of solution made from 2.4 moles of. Molarity And Dilution Worksheet Answers Chemistry.
From studyzonemeier.z13.web.core.windows.net
Molarity Worksheet Answers Chemistry Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Then use that as the initial concentration for the second. For chemistry help, visit www.chemfiesta.com. Molarity review problems 1) what is the. Molarity And Dilution Worksheet Answers Chemistry.
From quizzlistreplevies.z13.web.core.windows.net
Molarity And Dilution Worksheet Answers Chemistry Molarity And Dilution Worksheet Answers Chemistry Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as the initial concentration for the second. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in. Molarity And Dilution Worksheet Answers Chemistry.
From quizzlistreplevies.z13.web.core.windows.net
Molarity Worksheet 1 Answer Key Chemistry Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. First find the concentration of solution a. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. For chemistry help, visit www.chemfiesta.com. Calculate. Molarity And Dilution Worksheet Answers Chemistry.
From www.chegg.com
Solved Molarity And Dilutions Worksheet 1. What Is The Mo... Molarity And Dilution Worksheet Answers Chemistry Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. For chemistry help, visit www.chemfiesta.com. Then use that as the initial concentration for the second. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity.. Molarity And Dilution Worksheet Answers Chemistry.
From dokumen.tips
(PDF) CHEM 12 Practice Worksheet Molarity, Dilution & Dissociation · PDF fileCHEM 12 Practice Molarity And Dilution Worksheet Answers Chemistry For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate molarity by dissolving 25.0g naoh in 325 ml of. Molarity And Dilution Worksheet Answers Chemistry.
From www.showme.com
Molarity practice worksheet 13 Science, Chemistry, Solutions Chemistry ShowMe Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. For chemistry help, visit www.chemfiesta.com. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Then use that as the initial concentration for the second. Calculate molarity by dissolving. Molarity And Dilution Worksheet Answers Chemistry.
From www.judithcahen.net
Molarity And Dilution Phet Lab Answer Key » Judithcahen Answer Key For Practice Test Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. First find the concentration of solution a. For chemistry help, visit www.chemfiesta.com. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as the initial concentration for the second. Molarity review problems 1) what. Molarity And Dilution Worksheet Answers Chemistry.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Molarity And Dilution Worksheet Answers Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. What is the molarity of 2.0 l of solution made from. Molarity And Dilution Worksheet Answers Chemistry.
From studylib.net
Molarity Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration for the second. 1) if i have 340 ml of a 0.5 m nabr solution, what will. Molarity And Dilution Worksheet Answers Chemistry.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Molarity And Dilution Worksheet Answers Chemistry For chemistry help, visit www.chemfiesta.com. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then. Molarity And Dilution Worksheet Answers Chemistry.
From askworksheet.com
Molarity And Dilution Worksheet Askworksheet Molarity And Dilution Worksheet Answers Chemistry Then use that as the initial concentration for the second. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. For chemistry help, visit www.chemfiesta.com. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. First find the. Molarity And Dilution Worksheet Answers Chemistry.
From kidsworksheetfun.com
Molarity And Dilution Worksheet kidsworksheetfun Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. First find the concentration of solution a. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Molarity review problems 1) what is the molarity of. Molarity And Dilution Worksheet Answers Chemistry.
From www.studocu.com
Molarity and dilution worksheets 1 Molarity Problems Worksheet M = n n= moles V V must Molarity And Dilution Worksheet Answers Chemistry Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant,. Molarity And Dilution Worksheet Answers Chemistry.
From lessoncampusunspelt.z13.web.core.windows.net
Molarity Worksheet 1 Answer Key Chemistry Molarity And Dilution Worksheet Answers Chemistry Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. First find the concentration of solution a. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity. Molarity And Dilution Worksheet Answers Chemistry.
From www.chegg.com
Solved CHEM 101 Worksheet 11 molarity & dilution Name 1. Molarity And Dilution Worksheet Answers Chemistry First find the concentration of solution a. For chemistry help, visit www.chemfiesta.com. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration. Molarity And Dilution Worksheet Answers Chemistry.
From learningcampusstall.z21.web.core.windows.net
Molarity Worksheet 1 Answer Key Chemistry Molarity And Dilution Worksheet Answers Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. First find the concentration of solution a. Then use that as the initial concentration for the second. Add more solvent to a solution, thus volume of solution increases while. Molarity And Dilution Worksheet Answers Chemistry.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Worksheets Library Molarity And Dilution Worksheet Answers Chemistry Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as the initial concentration for the second. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. Calculate. Molarity And Dilution Worksheet Answers Chemistry.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Molarity And Dilution Worksheet Answers Chemistry Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration for the second. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. 1). Molarity And Dilution Worksheet Answers Chemistry.
From davida.davivienda.com
Molarity Dilution Worksheet Printable Word Searches Molarity And Dilution Worksheet Answers Chemistry Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as. Molarity And Dilution Worksheet Answers Chemistry.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Molarity And Dilution Worksheet Answers Chemistry For chemistry help, visit www.chemfiesta.com. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. First find the concentration of solution a. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration for the second. Calculate molarity. Molarity And Dilution Worksheet Answers Chemistry.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Molarity And Dilution Worksheet Answers Chemistry Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing the molarity. For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl. Molarity And Dilution Worksheet Answers Chemistry.
From studylib.net
Dilutions Worksheet 11UChem Molarity And Dilution Worksheet Answers Chemistry For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Then use that as the initial concentration. Molarity And Dilution Worksheet Answers Chemistry.
From printablelibswathed.z19.web.core.windows.net
Molarity And Dilution Worksheet Molarity And Dilution Worksheet Answers Chemistry Molarity review problems 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. For chemistry help, visit www.chemfiesta.com. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. First find the. Molarity And Dilution Worksheet Answers Chemistry.
From learningdbhimmel.z19.web.core.windows.net
Molarity Problems Worksheet With Answers Molarity And Dilution Worksheet Answers Chemistry Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as the initial concentration for the second. First find the concentration of solution a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. What is the molarity of 2.0 l of solution made from 2.4. Molarity And Dilution Worksheet Answers Chemistry.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Worksheets Library Molarity And Dilution Worksheet Answers Chemistry 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. For chemistry help, visit www.chemfiesta.com. First find the concentration of solution a. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Then use that as the initial concentration for the second. Molarity review problems 1) what is the. Molarity And Dilution Worksheet Answers Chemistry.