No2 Enthalpy Of Formation . Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The aggregate state is given in parentheses following the formula, such as: Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements:
from www.bartleby.com
The formation of any chemical can be as a reaction from the corresponding elements: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as:
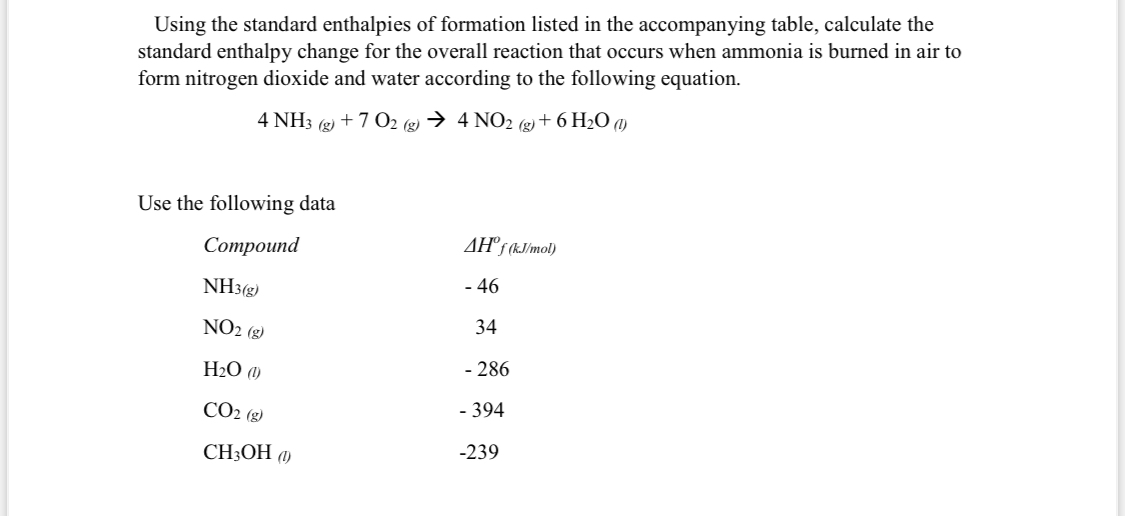
Answered Using the standard enthalpies of… bartleby
No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements: The aggregate state is given in parentheses following the formula, such as:
From www.meritnation.com
IF THE HEAT OF FORMATION OF NO2 IS X THE HEAT OF REACTION OF N2 +O2 No2 Enthalpy Of Formation The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The aggregate state is given in parentheses following the formula, such as: The formation of any chemical can be as a reaction from the corresponding elements: Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially.. No2 Enthalpy Of Formation.
From www.toppr.com
The enthalpies of formation of N2O and NO are 28 and 90 kJ mol^1 No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of. No2 Enthalpy Of Formation.
From mavink.com
Enthalpy Of Formation Equation No2 Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Selected atct [1, 2]. No2 Enthalpy Of Formation.
From www.numerade.com
SOLVED The enthalpy of of NO2Cl is 114.0 kJ. Use the No2 Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of. No2 Enthalpy Of Formation.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The aggregate state is given in parentheses following the formula, such as: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole. No2 Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free No2 Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol.. No2 Enthalpy Of Formation.
From brainly.in
Enthalpy of formation of NO2, SO2, Co2, and NH3,are 33.18, 296, 393 No2 Enthalpy Of Formation The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Enthalpy of formation (\(δh_f\)) is. No2 Enthalpy Of Formation.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby No2 Enthalpy Of Formation It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.. No2 Enthalpy Of Formation.
From slideplayer.com
Hess’ Law and enthalpy cycles using enthalpy of formation ppt download No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of the formation of. No2 Enthalpy Of Formation.
From www.numerade.com
The standard enthalpy of formation of NO2(g) and N2O4(g) are +33.18 kJ No2 Enthalpy Of Formation The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The aggregate state is given in parentheses following the formula, such as: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure. No2 Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of. No2 Enthalpy Of Formation.
From oneclass.com
OneClass Calculate the standard enthalpy of formation of N2O5 from the No2 Enthalpy Of Formation The formation of any chemical can be as a reaction from the corresponding elements: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Standard enthalpy change of formation (data table). No2 Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube No2 Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. The formation of any chemical can be as a. No2 Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation No2 Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of. No2 Enthalpy Of Formation.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The formation of any chemical can be as a reaction from the corresponding elements: It means that 33.2 kj of energy is required to form one. No2 Enthalpy Of Formation.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and No2 Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol.. No2 Enthalpy Of Formation.
From slideplayer.com
Chapter 2 The First Law. ppt download No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj. No2 Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy No2 Enthalpy Of Formation The formation of any chemical can be as a reaction from the corresponding elements: Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one. No2 Enthalpy Of Formation.
From www.numerade.com
SOLVED Calculate the Gibbs free energy for the formation of N2O4 gas No2 Enthalpy Of Formation The formation of any chemical can be as a reaction from the corresponding elements: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and. No2 Enthalpy Of Formation.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work No2 Enthalpy Of Formation The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol. No2 Enthalpy Of Formation.
From byjus.com
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110 No2 Enthalpy Of Formation It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements,. No2 Enthalpy Of Formation.
From www.toppr.com
If the heat of formation of NO2 is 'x' [1/2 N2(g) + O2(g)→ NO2(g)] the No2 Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. No2 Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole. No2 Enthalpy Of Formation.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c.. No2 Enthalpy Of Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole. No2 Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpies of Formation and Reaction PowerPoint Presentation ID No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of. No2 Enthalpy Of Formation.
From www.coursehero.com
[Solved] . 24. Construct an enthalpy diagram for the formation of NO2(g No2 Enthalpy Of Formation It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.. No2 Enthalpy Of Formation.
From mungfali.com
Enthalpies Of Formation Table No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one. No2 Enthalpy Of Formation.
From www.chegg.com
Solved Gaseous Nitrogen Dioxide NO2, Reacts With Oxygen T... No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25. No2 Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube No2 Enthalpy Of Formation It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2 ) and one mole of oxygen (o 2 ) at 1 atmospheric pressure and 25 ˚c. The formation of any chemical can be as a reaction from the corresponding elements: Selected atct [1, 2] enthalpy of formation. No2 Enthalpy Of Formation.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The aggregate state is given in parentheses following the formula, such as: The formation of any chemical. No2 Enthalpy Of Formation.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The aggregate state is given in parentheses following the formula, such as: The formation of any chemical can be as a reaction from the corresponding elements: It means that 33.2 kj of energy is required to form one mole. No2 Enthalpy Of Formation.
From mungfali.com
Enthalpies Of Formation Chart No2 Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct results was partially. The aggregate state is given in parentheses following the formula, such as: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of. No2 Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps No2 Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Selected atct [1, 2] enthalpy of formation. No2 Enthalpy Of Formation.
From www.toppr.com
The standard enthalpies of formation of NO2(g) and N2O4(g) 8 and 4 kcal No2 Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The aggregate state is given in parentheses following the formula, such as: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. No2 Enthalpy Of Formation.