What Does Q Represent In Calorimetry . It is calculated using the formula q = mcδt, where. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. For example, when an exothermic. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. In calorimetry, q represents the heat absorbed or released during a chemical reaction. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign.
from www.learnable.education
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. For example, when an exothermic. That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy.
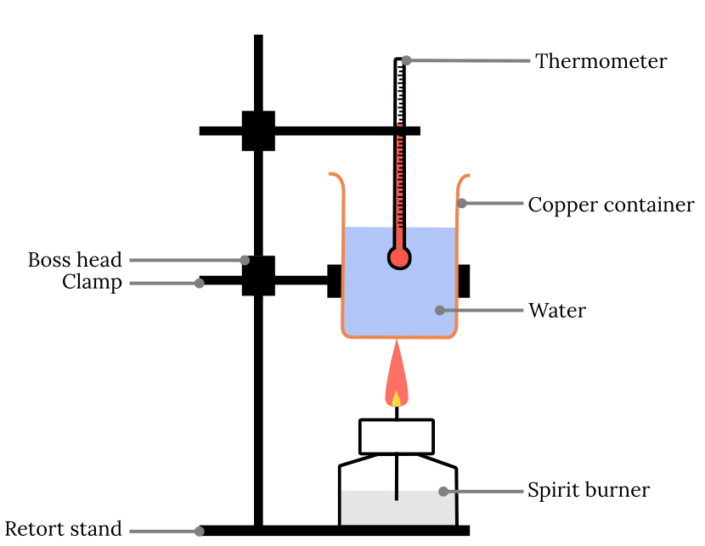
Year 11 Chemistry Practical Investigation Calorimetry Experiment
What Does Q Represent In Calorimetry The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. For example, when an exothermic. It is calculated using the formula q = mcδt, where. For example, when an exothermic. In calorimetry, q represents the heat absorbed or released during a chemical reaction. When q q is positive, the substance absorbs thermal energy and its. That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment What Does Q Represent In Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In calorimetry, q represents the heat absorbed or released during a chemical reaction. It is calculated using the formula q = mcδt, where. The amount of heat (q) (q) is positive or negative depending on whether. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Does Q Represent In Calorimetry Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the. What Does Q Represent In Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent What Does Q Represent In Calorimetry It is calculated using the formula q = mcδt, where. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. A calorimeter is a device used. What Does Q Represent In Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Does Q Represent In Calorimetry For example, when an exothermic. It is calculated using the formula q = mcδt, where. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same. What Does Q Represent In Calorimetry.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube What Does Q Represent In Calorimetry The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. For example, when an exothermic. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. The amount of heat (q) (q) is positive or negative depending on whether the. What Does Q Represent In Calorimetry.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube What Does Q Represent In Calorimetry It is calculated using the formula q = mcδt, where. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. In calorimetry, q represents the heat absorbed or released during a chemical reaction. The formula q = mcδt is used to calculate the. What Does Q Represent In Calorimetry.
From courses.lumenlearning.com
9.2 Calorimetry General College Chemistry I What Does Q Represent In Calorimetry The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. When q q is positive, the substance absorbs thermal energy and its. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. For example, when an exothermic. The value of c is. What Does Q Represent In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry What Does Q Represent In Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. For example, when an exothermic. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. In calorimetry, q represents the. What Does Q Represent In Calorimetry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Does Q Represent In Calorimetry When q q is positive, the substance absorbs thermal energy and its. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The amount of heat (q) (q) is. What Does Q Represent In Calorimetry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Does Q Represent In Calorimetry When q q is positive, the substance absorbs thermal energy and its. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The amount of heat (q) (q) is positive or negative depending on. What Does Q Represent In Calorimetry.
From studylib.net
Calorimetry What Does Q Represent In Calorimetry The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. For example, when an exothermic. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. That is, all the heat. What Does Q Represent In Calorimetry.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry What Does Q Represent In Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. For example, when an exothermic. When q q is positive, the substance absorbs thermal. What Does Q Represent In Calorimetry.
From sites.google.com
Calorimetry Preliminary HSC Chemistry What Does Q Represent In Calorimetry It is calculated using the formula q = mcδt, where. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. The formula q = mcδt is used to calculate the heat energy. What Does Q Represent In Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube What Does Q Represent In Calorimetry That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. For example, when an exothermic. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. A calorimeter is. What Does Q Represent In Calorimetry.
From www.youtube.com
AP Chemistry Calorimetry Basics and worksheet review YouTube What Does Q Represent In Calorimetry That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The value of c. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Does Q Represent In Calorimetry When q q is positive, the substance absorbs thermal energy and its. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. That is, all the heat exchanged with the reaction. What Does Q Represent In Calorimetry.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations What Does Q Represent In Calorimetry It is calculated using the formula q = mcδt, where. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. For example, when an exothermic. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have. What Does Q Represent In Calorimetry.
From studylib.net
Calorimetry What Does Q Represent In Calorimetry That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. For example, when an exothermic. For example, when an exothermic. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. A calorimeter is a device used to measure the amount of. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Does Q Represent In Calorimetry The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. For example, when an exothermic. That is, all the heat exchanged with the reaction is absorbed or released by. What Does Q Represent In Calorimetry.
From users.highland.edu
Calorimetry What Does Q Represent In Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy.. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 What Does Q Represent In Calorimetry That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. In calorimetry, q represents the heat absorbed or released during a chemical reaction. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. For example, when an exothermic. A calorimeter is a device used. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Does Q Represent In Calorimetry The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure. What Does Q Represent In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Does Q Represent In Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. It is calculated using the formula q = mcδt,. What Does Q Represent In Calorimetry.
From www.youtube.com
050 Calorimetry YouTube What Does Q Represent In Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. When q q is positive, the substance absorbs thermal energy and its. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. It is calculated using the formula q = mcδt, where. The value of c is. What Does Q Represent In Calorimetry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog What Does Q Represent In Calorimetry When q q is positive, the substance absorbs thermal energy and its. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. That is, all the heat exchanged with the reaction. What Does Q Represent In Calorimetry.
From slideplayer.com
LECTURE 8.4 CALORIMETRY. ppt download What Does Q Represent In Calorimetry For example, when an exothermic. For example, when an exothermic. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. A calorimeter is a device used to measure the. What Does Q Represent In Calorimetry.
From studylib.net
PPT Calorimetry What Does Q Represent In Calorimetry Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. For example, when an exothermic.. What Does Q Represent In Calorimetry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube What Does Q Represent In Calorimetry It is calculated using the formula q = mcδt, where. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. The value of c is intrinsically a positive number, but δt and q can be either positive. What Does Q Represent In Calorimetry.
From www.slideshare.net
Lesson Enthalpy and Calorimetry What Does Q Represent In Calorimetry In calorimetry, q represents the heat absorbed or released during a chemical reaction. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. That is, all the heat exchanged with the reaction is absorbed or released by the calorimeter and its contents. The amount of heat (q) (q) is. What Does Q Represent In Calorimetry.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab What Does Q Represent In Calorimetry The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Does Q Represent In Calorimetry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Does Q Represent In Calorimetry For example, when an exothermic. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. It is calculated using the formula q = mcδt, where. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used. What Does Q Represent In Calorimetry.
From studylib.net
Calorimetry What Does Q Represent In Calorimetry In calorimetry, q represents the heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. The value of c is intrinsically a positive number, but δt. What Does Q Represent In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Does Q Represent In Calorimetry The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. The value of c is intrinsically a. What Does Q Represent In Calorimetry.
From guweb2.gonzaga.edu
CHEM 101 General Chemistry topic What Does Q Represent In Calorimetry Q reaction is the enthalpy of reaction (\(\delta h_{r}\)) for a system at constant. In calorimetry, q represents the heat absorbed or released during a chemical reaction. It is calculated using the formula q = mcδt, where. The amount of heat (q) (q) is positive or negative depending on whether the substance absorbs or releases thermal energy. A calorimeter is. What Does Q Represent In Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Does Q Represent In Calorimetry For example, when an exothermic. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry experiment. For example, when an exothermic. The magnitude of the temperature change depends on both the amount of thermal energy transferred (q) and the heat capacity of. When q q is positive, the substance absorbs thermal energy and its.. What Does Q Represent In Calorimetry.