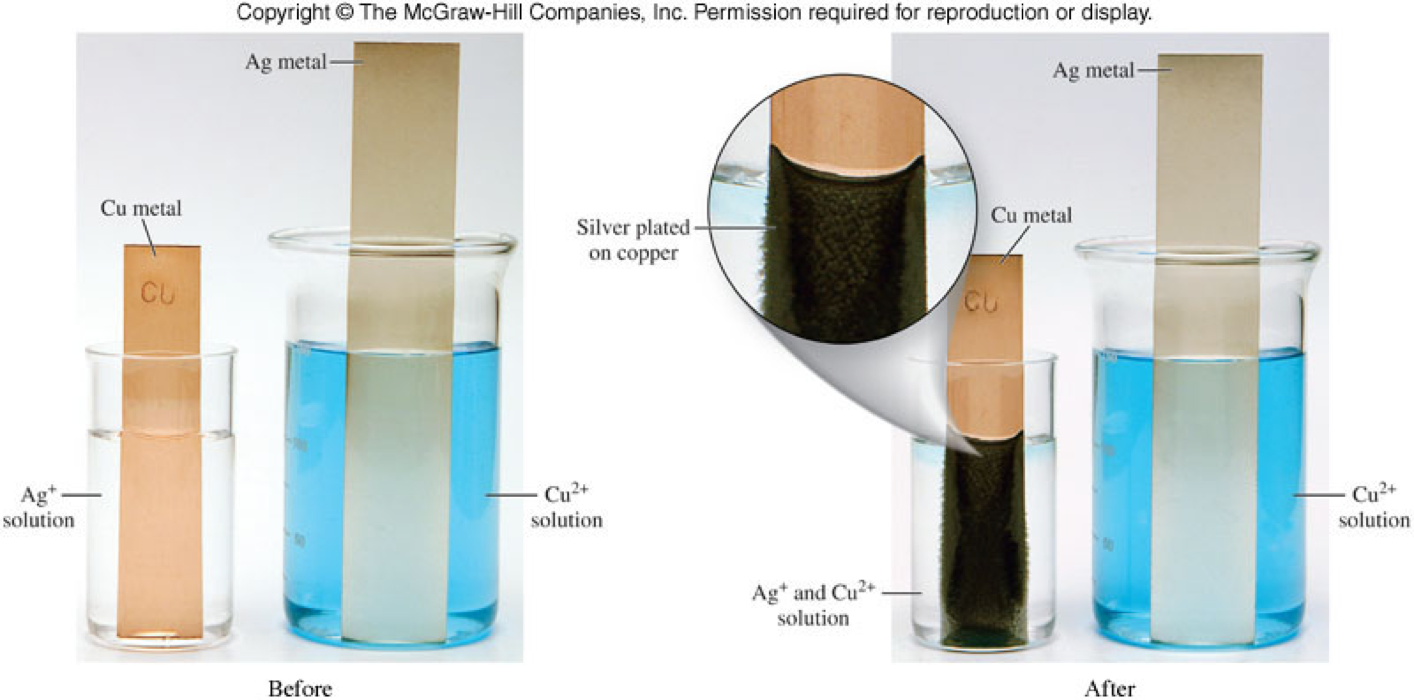
Copper Metal In Silver Nitrate Solution . Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. As it does so, the silver in solution will. A spiral of copper metal is placed in a solution of silver nitrate. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Copper nitrate is seen turning the. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Crystals of silver will start to grow. The solution gradually acquires the blue. Metallic copper is placed in a solution of silver nitrate. Silver metal forms at the surface of the copper metal.
from shaunmwilliams.com
As it does so, the silver in solution will. Copper nitrate is seen turning the. The solution gradually acquires the blue. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Metallic copper is placed in a solution of silver nitrate. Crystals of silver will start to grow. Silver metal forms at the surface of the copper metal. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope.
Lecture 5 Presentation
Copper Metal In Silver Nitrate Solution Metallic copper is placed in a solution of silver nitrate. Crystals of silver will start to grow. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Metallic copper is placed in a solution of silver nitrate. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Silver metal forms at the surface of the copper metal. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Copper nitrate is seen turning the. The solution gradually acquires the blue. A spiral of copper metal is placed in a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate.
From www.youtube.com
Demonstration Displacement Reaction of Silver Nitrate and Copper Metal Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. Silver metal forms at the surface of the copper metal. A spiral of copper metal is placed in a solution of silver nitrate. Copper nitrate is seen turning the. Crystals of silver will start to grow. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. The reaction of. Copper Metal In Silver Nitrate Solution.
From www.toppr.com
Vidisha placed a copper wire in silver nitrate solution as shown in the Copper Metal In Silver Nitrate Solution Silver metal forms at the surface of the copper metal. A spiral of copper metal is placed in a solution of silver nitrate. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. The solution gradually acquires the blue. Overnight, the. Copper Metal In Silver Nitrate Solution.
From www.alamy.com
Reaction of a rod of copper in a solution of silver nitrate.rs Stock Copper Metal In Silver Nitrate Solution The solution gradually acquires the blue. Crystals of silver will start to grow. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Metallic copper is placed in a solution of silver nitrate. As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry. Copper Metal In Silver Nitrate Solution.
From www.alamy.com
Copper coin being coated with silver crystals in a solution of silver Copper Metal In Silver Nitrate Solution Metallic copper is placed in a solution of silver nitrate. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Copper nitrate is seen turning the. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. The solution gradually acquires the blue. Silver metal forms at the surface of. Copper Metal In Silver Nitrate Solution.
From www.reddit.com
Copper wire & silver nitrate solution r/VisualChemistry Copper Metal In Silver Nitrate Solution The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Crystals of silver will start to grow. The solution gradually acquires the blue. Metallic copper is placed in a solution of silver nitrate. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Cu + agno3 = cu(no3)2 + ag is. Copper Metal In Silver Nitrate Solution.
From www.slideserve.com
PPT Reaction of silver nitrate with copper to make silver and copper Copper Metal In Silver Nitrate Solution Crystals of silver will start to grow. Copper nitrate is seen turning the. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Silver metal forms at the surface of the copper metal. The solution gradually acquires the blue. A spiral of copper metal is placed in a solution of silver nitrate. A simple. Copper Metal In Silver Nitrate Solution.
From www.alamy.com
copper displacing silver from silver nitrate solution Stock Photo Alamy Copper Metal In Silver Nitrate Solution The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Metallic copper is placed in a solution of silver nitrate. Silver metal forms at the surface of the copper metal. A spiral of copper metal is placed in a solution of silver nitrate. The solution gradually acquires the blue. Cu + agno3 = cu(no3)2 + ag. Copper Metal In Silver Nitrate Solution.
From www.sciencephoto.com
Silver nitratecopper displacement reaction Stock Image C002/7894 Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. A spiral of copper metal is placed in a solution of silver nitrate. The silver nitrate is in solution and the metallic copper will dissolve to form. Copper Metal In Silver Nitrate Solution.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Copper Metal In Silver Nitrate Solution Metallic copper is placed in a solution of silver nitrate. The solution gradually acquires the blue. As it does so, the silver in solution will. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The silver nitrate is in solution and the metallic copper will. Copper Metal In Silver Nitrate Solution.
From sciencephoto.com
Copper in Silver Nitrate Stock Image C002/7943 Science Photo Library Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Silver metal forms at the surface of the copper metal. Copper nitrate is seen turning the. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Crystals of silver will start to grow. Carefully add. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Copper Metal In Silver Nitrate Solution A spiral of copper metal is placed in a solution of silver nitrate. Crystals of silver will start to grow. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. The silver nitrate is. Copper Metal In Silver Nitrate Solution.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Copper Metal In Silver Nitrate Solution The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Metallic copper is placed in a solution of silver nitrate. Crystals of silver will start to grow. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs Copper Metal In Silver Nitrate Solution The solution gradually acquires the blue. As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Crystals of silver will start to grow.. Copper Metal In Silver Nitrate Solution.
From brainly.in
why the solution of silver nitrate blue in colour after Copper Metal In Silver Nitrate Solution Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Copper nitrate is seen turning the. A simple redox reaction occurs when copper metal is immersed in a solution of silver. Copper Metal In Silver Nitrate Solution.
From www.alamy.com
Displacement reaction of copper and silver nitrate solution. When Copper Metal In Silver Nitrate Solution A spiral of copper metal is placed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Carefully add one drop of. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Crystals of silver will start to grow. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction. Copper Metal In Silver Nitrate Solution.
From shaunmwilliams.com
Lecture 5 Presentation Copper Metal In Silver Nitrate Solution The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Crystals of silver will start to grow. Metallic copper is placed in a solution of silver nitrate. Copper nitrate is seen turning the. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Metal In Silver Nitrate Solution Copper nitrate is seen turning the. Crystals of silver will start to grow. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. A spiral of copper metal is placed in a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two. Copper Metal In Silver Nitrate Solution.
From www.youtube.com
Copper and Silver Nitrate solution time lapse YouTube Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. A spiral of copper metal is placed in a solution of silver nitrate. Copper nitrate is seen turning the. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. The silver nitrate is. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Metal In Silver Nitrate Solution Crystals of silver will start to grow. Silver metal forms at the surface of the copper metal. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Copper nitrate is seen turning the. Metallic. Copper Metal In Silver Nitrate Solution.
From uwaterloo.ca
Copper wire in a silver nitrate solution Chem 13 News Magazine Copper Metal In Silver Nitrate Solution The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. The solution gradually acquires the blue. Crystals of silver will start to grow. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Metallic copper is placed in. Copper Metal In Silver Nitrate Solution.
From www.reddit.com
Single replacement reaction Copper in a Silver nitrate solution. r Copper Metal In Silver Nitrate Solution A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Silver metal forms at the surface of the copper metal. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; As it does so,. Copper Metal In Silver Nitrate Solution.
From www.numerade.com
The reaction between solid copper and aqueous silver nitrate produces Copper Metal In Silver Nitrate Solution The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. A spiral of copper metal is placed in a solution of silver nitrate. Crystals of silver will start to grow. Cu +. Copper Metal In Silver Nitrate Solution.
From www.alamy.com
Copper silver nitrate solution hires stock photography and images Alamy Copper Metal In Silver Nitrate Solution Overnight, the copper dissolves, silver is precipitated and the solution turns blue. The solution gradually acquires the blue. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in. Copper Metal In Silver Nitrate Solution.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 5 of 6 Stock Image C028/1083 Copper Metal In Silver Nitrate Solution Crystals of silver will start to grow. A spiral of copper metal is placed in a solution of silver nitrate. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; As it does so, the silver in solution will. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Copper. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs Copper Metal In Silver Nitrate Solution The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; As it does so, the silver in solution will. The solution gradually acquires the blue. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Copper nitrate is seen turning the. A spiral of copper metal is placed in. Copper Metal In Silver Nitrate Solution.
From www.chegg.com
Solved Copper can displace silver out of silver nitrate Copper Metal In Silver Nitrate Solution The solution gradually acquires the blue. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. A spiral of copper metal is placed in a solution of silver nitrate. Copper nitrate is seen turning. Copper Metal In Silver Nitrate Solution.
From www.youtube.com
Balance AgNO3 + Cu = Cu(NO3)2 + Ag (Silver Nitrate and Copper) YouTube Copper Metal In Silver Nitrate Solution The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Crystals of silver will start to grow. Metallic copper is placed in a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction. Copper Metal In Silver Nitrate Solution.
From www.youtube.com
CBSE Class 10 Chemistry Copper reacts with Silver Nitrate Solution Copper Metal In Silver Nitrate Solution Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. As it does so, the silver. Copper Metal In Silver Nitrate Solution.
From shaunmwilliams.com
Lecture 5 Presentation Copper Metal In Silver Nitrate Solution A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Overnight, the copper dissolves, silver. Copper Metal In Silver Nitrate Solution.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs Copper Metal In Silver Nitrate Solution As it does so, the silver in solution will. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; Silver metal forms at the surface of the copper metal. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Crystals of silver will start to grow. The reaction of. Copper Metal In Silver Nitrate Solution.
From brainly.in
In the refining of silver, the recovery of silver from silver nitrate Copper Metal In Silver Nitrate Solution The solution gradually acquires the blue. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Carefully add one drop of silver nitrate solution to the copper and refocus the microscope. Silver metal forms at the surface of the copper metal. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. Metallic copper is. Copper Metal In Silver Nitrate Solution.
From www.doubtnut.com
When a copper rod is dipped in aqueous silver nitrate solution , the c Copper Metal In Silver Nitrate Solution Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Copper nitrate is seen turning the. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; The solution gradually acquires the blue. Overnight, the copper dissolves, silver is precipitated and. Copper Metal In Silver Nitrate Solution.
From ar.inspiredpencil.com
Silver Nitrate Solution Copper Metal In Silver Nitrate Solution Silver metal forms at the surface of the copper metal. A spiral of copper metal is placed in a solution of silver nitrate. Metallic copper is placed in a solution of silver nitrate. The solution gradually acquires the blue. The silver nitrate is in solution and the metallic copper will dissolve to form copper nitrate; The reaction of copper wire. Copper Metal In Silver Nitrate Solution.
From www.sciencephoto.com
Copper reacting with silver nitrate Stock Image C030/8167 Science Copper Metal In Silver Nitrate Solution Metallic copper is placed in a solution of silver nitrate. Overnight, the copper dissolves, silver is precipitated and the solution turns blue. A spiral of copper metal is placed in a solution of silver nitrate. The solution gradually acquires the blue. Silver metal forms at the surface of the copper metal. A simple redox reaction occurs when copper metal is. Copper Metal In Silver Nitrate Solution.