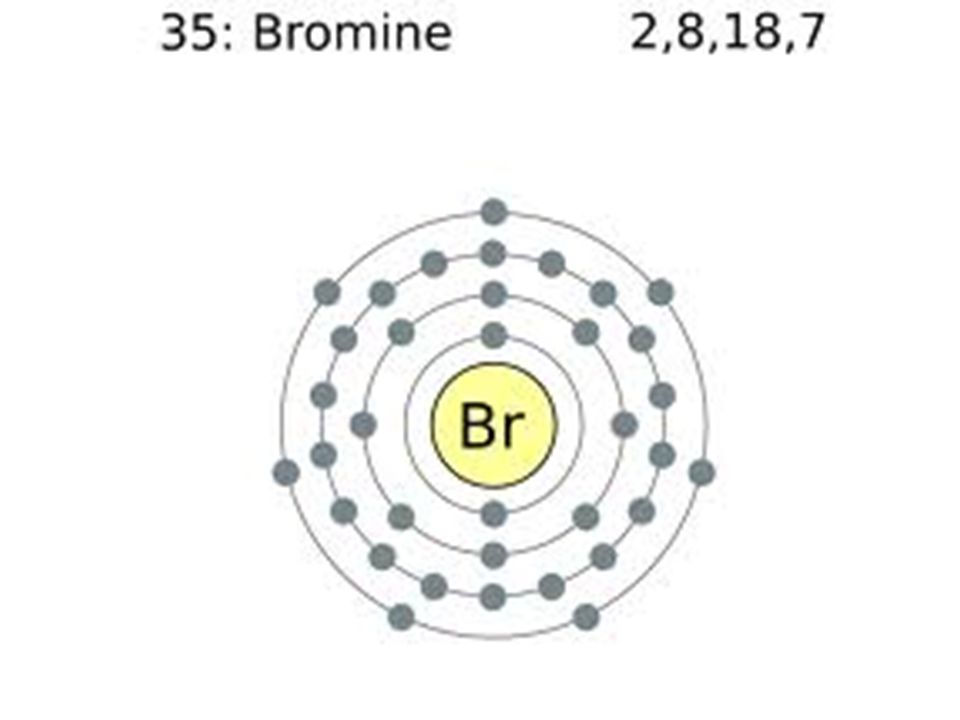
Electron Configuration Of Bromine Ion . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. This can be shortened to [ar]4s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Justify the observed charge of ions to their electronic configuration. General rules of electron configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Determine the electron configuration of ions. The bromide ion has the same electron configuration as a neutral bromine. Use a chart such as the one below.
from periodictable.me
The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Justify the observed charge of ions to their electronic configuration. There are a set of general rules that are used to figure out the electron configuration of an atomic species: General rules of electron configuration. The bromide ion has the same electron configuration as a neutral bromine. Use a chart such as the one below. Determine the electron configuration of ions. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
Bromine Electron Configuration (Br) with Orbital Diagram
Electron Configuration Of Bromine Ion The bromide ion has the same electron configuration as a neutral bromine. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The bromide ion has the same electron configuration as a neutral bromine. This can be shortened to [ar]4s23d104p5. General rules of electron configuration. Use a chart such as the one below. Justify the observed charge of ions to their electronic configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Determine the electron configuration of ions. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species:
From www.alamy.com
3d render of atom structure of bromine isolated over white background Electron Configuration Of Bromine Ion There are a set of general rules that are used to figure out the electron configuration of an atomic species: Justify the observed charge of ions to their electronic configuration. Use a chart such as the one below. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. General rules of electron configuration. The bromide ion has. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion Justify the observed charge of ions to their electronic configuration. General rules of electron configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The bromide ion has the same electron configuration as a neutral bromine.. Electron Configuration Of Bromine Ion.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Electron Configuration Of Bromine Ion Justify the observed charge of ions to their electronic configuration. This can be shortened to [ar]4s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Determine the electron configuration of ions. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Use a chart such as the one below.. Electron Configuration Of Bromine Ion.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Electron Configuration Of Bromine Ion The bromide ion has the same electron configuration as a neutral bromine. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Use a chart such as the one below. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Determine the electron configuration of ions. Justify. Electron Configuration Of Bromine Ion.
From material-properties.org
Bromine Periodic Table and Atomic Properties Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below. This can be shortened to [ar]4s23d104p5. The electron configuration of the bromide. Electron Configuration Of Bromine Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below. This can be shortened to [ar]4s23d104p5. Justify the observed charge of ions to their electronic configuration. There are a set of general rules that are used to figure out the electron configuration. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion This can be shortened to [ar]4s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The bromide ion has the same electron configuration as a neutral bromine. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Justify the observed charge of ions to their electronic. Electron Configuration Of Bromine Ion.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromide ion has the same electron configuration as a neutral bromine. Justify the observed charge of ions to their electronic configuration. This can be shortened to [ar]4s23d104p5.. Electron Configuration Of Bromine Ion.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration Of Bromine Ion Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. This can be shortened to [ar]4s23d104p5. Determine the electron configuration of ions. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Use a chart such. Electron Configuration Of Bromine Ion.
From ar.inspiredpencil.com
Bromine Valence Electrons Electron Configuration Of Bromine Ion There are a set of general rules that are used to figure out the electron configuration of an atomic species: Use a chart such as the one below. This can be shortened to [ar]4s23d104p5. General rules of electron configuration. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromide. Electron Configuration Of Bromine Ion.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Electron Configuration Of Bromine Ion Use a chart such as the one below. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. General rules of electron configuration. Justify the observed charge of ions to their electronic configuration.. Electron Configuration Of Bromine Ion.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Electron Configuration Of Bromine Ion Determine the electron configuration of ions. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Justify the observed charge of ions to their electronic configuration. There are a set of general rules that are used to figure out the electron configuration of an atomic. Electron Configuration Of Bromine Ion.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Electron Configuration Of Bromine Ion The bromide ion has the same electron configuration as a neutral bromine. Justify the observed charge of ions to their electronic configuration. Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: General rules of. Electron Configuration Of Bromine Ion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Electron Configuration Of Bromine Ion This can be shortened to [ar]4s23d104p5. The bromide ion has the same electron configuration as a neutral bromine. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Use a chart such as the one below. General rules of electron configuration. Justify the observed charge of ions to their electronic configuration. There are a set of general. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion General rules of electron configuration. Determine the electron configuration of ions. Use a chart such as the one below. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The electron configuration of the bromide ion is. Electron Configuration Of Bromine Ion.
From www.pinterest.com
Bromine Facts Atomic Number 35 and Element Symbol Br Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The bromide ion has the same electron configuration as a neutral bromine. Use a chart such as the one below. Justify the observed charge of ions to their electronic configuration. General rules of electron configuration. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6.. Electron Configuration Of Bromine Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Configuration Of Bromine Ion Use a chart such as the one below. Justify the observed charge of ions to their electronic configuration. General rules of electron configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. This can be shortened to [ar]4s23d104p5. The bromide ion has the same. Electron Configuration Of Bromine Ion.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock Electron Configuration Of Bromine Ion There are a set of general rules that are used to figure out the electron configuration of an atomic species: This can be shortened to [ar]4s23d104p5. Determine the electron configuration of ions. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Justify the observed charge of ions to their electronic configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Electron Configuration Of Bromine Ion.
From ar.inspiredpencil.com
Electron Configuration For Bromine Electron Configuration Of Bromine Ion The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromide ion has the same electron configuration as a neutral bromine. Determine the electron configuration of ions. This can be shortened to [ar]4s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because. Electron Configuration Of Bromine Ion.
From www.youtube.com
Br electronic configurationHow to write electronic configuration of Electron Configuration Of Bromine Ion The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Justify the observed charge of ions to their electronic configuration. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Determine the electron configuration of ions. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration of the. Electron Configuration Of Bromine Ion.
From stock.adobe.com
Symbol and electron diagram for Bromine vector de Stock Adobe Stock Electron Configuration Of Bromine Ion The bromide ion has the same electron configuration as a neutral bromine. This can be shortened to [ar]4s23d104p5. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Determine the electron configuration of ions. There are a set of general rules that are used to figure out the electron configuration of an atomic species: $$\mathrm{1s^2 2s^2 2p^6 3s^2. Electron Configuration Of Bromine Ion.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Electron Configuration Of Bromine Ion Determine the electron configuration of ions. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. General rules of electron configuration. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Use a chart such as the one below. Find the full electronic configuration and valence electrons of. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Justify the observed charge of ions to their electronic configuration. This can be shortened to [ar]4s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The bromide ion has the same electron configuration as a neutral bromine. General rules of. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. General rules of electron configuration. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Determine the electron configuration of ions. There are a set of general. Electron Configuration Of Bromine Ion.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Electron Configuration Of Bromine Ion There are a set of general rules that are used to figure out the electron configuration of an atomic species: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Use a chart such as the one below. This can be shortened to [ar]4s23d104p5. The bromide ion has the same electron configuration as. Electron Configuration Of Bromine Ion.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Electron Configuration Of Bromine Ion Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The bromide ion has the same electron configuration as a neutral bromine. General rules of electron configuration. Justify the observed charge of ions to their electronic configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Determine the electron. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. This can be shortened to [ar]4s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The bromide ion has the same electron configuration as a neutral bromine. Determine the electron configuration of ions. Use a chart such as the one. Electron Configuration Of Bromine Ion.
From mavink.com
Lewis Dot Diagram For Bromine Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Justify the observed charge of ions to their electronic configuration. General rules of electron configuration. The electron configuration of the bromide ion is. Electron Configuration Of Bromine Ion.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Electron Configuration Of Bromine Ion The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. This can be shortened to [ar]4s23d104p5. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Determine the electron configuration of ions. There are a set. Electron Configuration Of Bromine Ion.
From www.animalia-life.club
Electron Configuration For Bromine Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Determine the electron configuration of ions. The bromide ion has. Electron Configuration Of Bromine Ion.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Electron Configuration Of Bromine Ion $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. There are a set of general rules that are used to figure out the electron configuration of an atomic species: The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. This can be shortened to [ar]4s23d104p5. Determine the. Electron Configuration Of Bromine Ion.
From ar.inspiredpencil.com
Electron Configuration For Bromine Electron Configuration Of Bromine Ion Determine the electron configuration of ions. Use a chart such as the one below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. General rules of electron configuration. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. Justify the observed charge of ions to their electronic configuration. This. Electron Configuration Of Bromine Ion.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Electron Configuration Of Bromine Ion Use a chart such as the one below. There are a set of general rules that are used to figure out the electron configuration of an atomic species: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. This can be shortened to [ar]4s23d104p5. General rules of electron configuration. The bromide ion has. Electron Configuration Of Bromine Ion.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Electron Configuration Of Bromine Ion Determine the electron configuration of ions. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Justify the observed charge of ions to their. Electron Configuration Of Bromine Ion.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Electron Configuration Of Bromine Ion There are a set of general rules that are used to figure out the electron configuration of an atomic species: The bromide ion has the same electron configuration as a neutral bromine. Justify the observed charge of ions to their electronic configuration. Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. $$\mathrm{1s^2 2s^2 2p^6. Electron Configuration Of Bromine Ion.