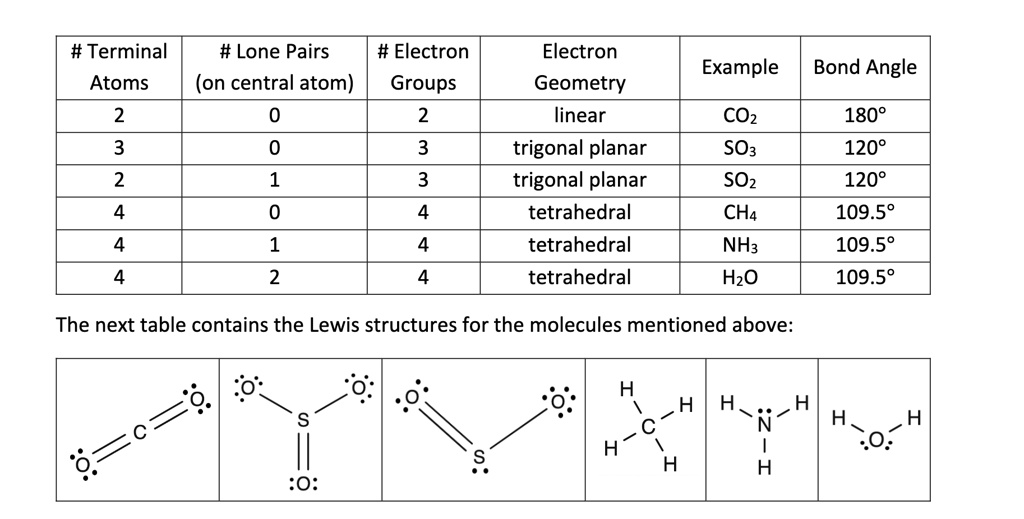
Terminal Atoms Examples . Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. This is the complete lewis structure of co 2. A lewis symbol consists of an elemental symbol surrounded by one dot for each. These four electrons can be. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Moving one lone pair from each terminal o atom, the following structure is obtained. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule.
from www.numerade.com
We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. This is the complete lewis structure of co 2. Moving one lone pair from each terminal o atom, the following structure is obtained. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. A lewis symbol consists of an elemental symbol surrounded by one dot for each. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. These four electrons can be. A lewis symbol consists of an elemental symbol surrounded by one dot for each.
SOLVED Terminal Lone Pairs Electron Atoms (on central atom) Groups
Terminal Atoms Examples Moving one lone pair from each terminal o atom, the following structure is obtained. This is the complete lewis structure of co 2. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. These four electrons can be. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Moving one lone pair from each terminal o atom, the following structure is obtained. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions.
From socratic.org
What part of an atom is involved in nuclear reactions? Socratic Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. This is the complete lewis structure. Terminal Atoms Examples.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Terminal Atoms Examples This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. A lewis symbol consists of an elemental symbol surrounded by one. Terminal Atoms Examples.
From studylib.net
Lewis Structure Terminal Atoms Examples Moving one lone pair from each terminal o atom, the following structure is obtained. A lewis symbol consists of an elemental symbol surrounded by one dot for each. These four electrons can be. This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom. Terminal Atoms Examples.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two. Terminal Atoms Examples.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Terminal Atoms Examples Moving one lone pair from each terminal o atom, the following structure is obtained. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Beginning with the terminal atoms, add. Terminal Atoms Examples.
From www.numerade.com
SOLVED Terminal Lone Pairs Electron Atoms (on central atom) Groups Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. A lewis symbol consists of an elemental symbol surrounded by one dot for each. Moving one lone pair from each terminal o atom, the following structure is obtained. These four electrons can be. For example, each atom of a group 14 element has four electrons in. Terminal Atoms Examples.
From in.pinterest.com
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons Terminal Atoms Examples This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. These four electrons can be. Beginning with the terminal. Terminal Atoms Examples.
From www.teachoo.com
What is Atom? How does it Exist? and it's Symbols Teachoo Terminal Atoms Examples Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. These four electrons can be. A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group. Terminal Atoms Examples.
From topglobenews.com
A Brief History of the Atom Top Globe News Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four. Terminal Atoms Examples.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Moving one lone pair from each terminal o atom, the following structure is obtained. These four electrons can be. For example, each atom of a group 14 element has four electrons in. Terminal Atoms Examples.
From www.slideserve.com
PPT Chapter 9 Molecular Structures PowerPoint Presentation, free Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. These four electrons can be. A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Lewis. Terminal Atoms Examples.
From www.slideserve.com
PPT Atoms Molecules Elements Compounds PowerPoint Presentation ID Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four. Terminal Atoms Examples.
From bgbasicscience.blogspot.com
Lets Get Inside An Atom!! The Science Station Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. Moving one lone pair from each terminal o atom, the following structure is obtained. A lewis symbol consists of an elemental symbol surrounded by one dot for each. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way. Terminal Atoms Examples.
From chemistryclinic.co.uk
Shapes of simple molecules Chemistry Terminal Atoms Examples For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen).. Terminal Atoms Examples.
From mumleyscience.weebly.com
The Atom Mumley Science Terminal Atoms Examples Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. This is the complete lewis structure of co 2. For example, each atom of a group 14 element has four electrons in its outermost shell and. Terminal Atoms Examples.
From www.storyboardthat.com
Atom Diagram What are Isotopes and Parts of an Atom Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. Moving one lone pair from each terminal o atom, the following structure. Terminal Atoms Examples.
From www.researchgate.net
Scheme 4. Actual number of examples and polarity of terminal atoms in Terminal Atoms Examples Moving one lone pair from each terminal o atom, the following structure is obtained. This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to. Terminal Atoms Examples.
From sites.google.com
Structure of the Atom Unit G Review Terminal Atoms Examples This is the complete lewis structure of co 2. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For example, each atom of a group 14 element has four electrons in its outermost shell and. Terminal Atoms Examples.
From leverageedu.com
Structure Of An Atom Class 9 Science Notes Leverage Edu Terminal Atoms Examples Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet.. Terminal Atoms Examples.
From study.com
Atoms vs. Molecule Definition, Differences & Characteristics Lesson Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These four electrons can be. A lewis symbol consists of an elemental symbol surrounded by one dot for each. This is the complete lewis structure of. Terminal Atoms Examples.
From kidspressmagazine.com
The Structure of Atoms Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. These four electrons can be. Moving one lone pair from each terminal o atom, the following structure is obtained. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic. Terminal Atoms Examples.
From www.chemicals.co.uk
Chemistry GCSE Revision Atomic Structure And The Periodic Table Terminal Atoms Examples Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. A lewis symbol consists of an elemental symbol surrounded by one dot for each. A lewis symbol consists of an elemental symbol surrounded by one dot. Terminal Atoms Examples.
From www.vedantu.com
Basic Structure of Atom Learn Definition, Facts and Examples Terminal Atoms Examples Moving one lone pair from each terminal o atom, the following structure is obtained. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. These four electrons can be. Beginning. Terminal Atoms Examples.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. Beginning with the terminal atoms, add enough electrons to each atom to. Terminal Atoms Examples.
From manuallistcantabank.z21.web.core.windows.net
Diagram Of The Atomic Structure Terminal Atoms Examples Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. A lewis symbol consists of an elemental symbol surrounded by one dot. Terminal Atoms Examples.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Terminal Atoms Examples This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). Moving one lone pair from each terminal o atom, the following structure is obtained. Lewis structure, also. Terminal Atoms Examples.
From hubpages.com
Atoms and Atomic Structure hubpages Terminal Atoms Examples For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). We use lewis symbols to describe valence electron configurations of atoms and monatomic ions.. Terminal Atoms Examples.
From chyscience.blogspot.com
Chemical Science Valence Shell Electron Pair Repulsion Theory Terminal Atoms Examples For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. This is the complete lewis structure. Terminal Atoms Examples.
From www.scienceabc.com
Isotopes Definition, Explanation, Properties And Examples Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. These four electrons can be. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple. Terminal Atoms Examples.
From studylib.net
Electron Groups on Central Atom Terminal Atoms Examples This is the complete lewis structure of co 2. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. Moving one lone. Terminal Atoms Examples.
From www.coursehero.com
Molecular Geometry Boundless Chemistry Course Hero Terminal Atoms Examples A lewis symbol consists of an elemental symbol surrounded by one dot for each. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two. Terminal Atoms Examples.
From sciencenotes.org
Learn the Parts of an Atom Terminal Atoms Examples Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet.. Terminal Atoms Examples.
From www.slideserve.com
PPT Lewis Dot Structures PowerPoint Presentation, free download ID Terminal Atoms Examples For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). Lewis structure, also known as lewis dot structure or electron dot structure, is a. Terminal Atoms Examples.
From mmerevise.co.uk
Atoms Questions and Revision MME Terminal Atoms Examples These four electrons can be. A lewis symbol consists of an elemental symbol surrounded by one dot for each. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. A lewis symbol consists of an elemental symbol surrounded by one dot for each. We. Terminal Atoms Examples.
From www.biologyonline.com
Atom Definition and Examples Biology Online Dictionary Terminal Atoms Examples We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. A lewis symbol consists of an elemental symbol surrounded by one dot for each. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). Lewis structure, also known as lewis dot structure or electron dot structure,. Terminal Atoms Examples.