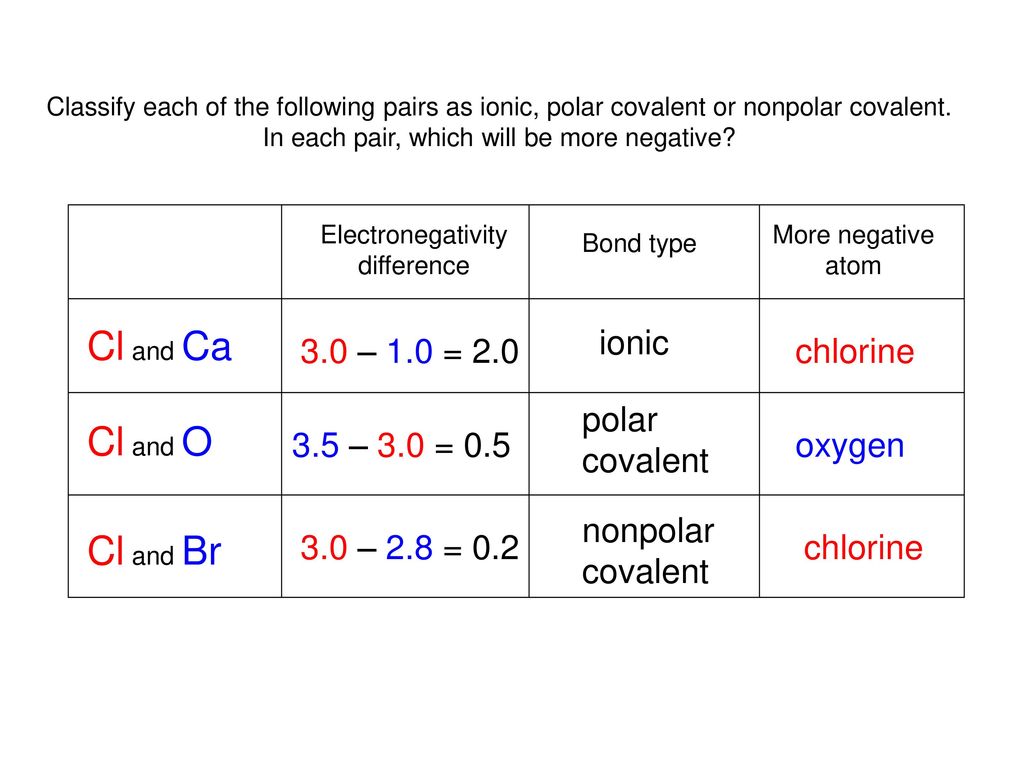
Chlorine And Carbon Electronegativity Difference . The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. What happens if two atoms of equal electronegativity bond together? Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. You can also use our tool as an.
from slideplayer.com
Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Why does electronegativity increase across a period? Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. What happens if two atoms of equal electronegativity bond together? Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. You can also use our tool as an. 119 rows values for electronegativity run from 0 to 4. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding.
Chemical Bonding. ppt download
Chlorine And Carbon Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). What happens if two atoms of equal electronegativity bond together? Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Why does electronegativity increase across a period? Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. 119 rows values for electronegativity run from 0 to 4. You can also use our tool as an.
From mungfali.com
Electronegativity Difference Chart Chlorine And Carbon Electronegativity Difference 119 rows values for electronegativity run from 0 to 4. Why does electronegativity increase across a period? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electronegativity calculator allows you to calculate. Chlorine And Carbon Electronegativity Difference.
From mungfali.com
Periodic Table Of Elements Electronegativity Chlorine And Carbon Electronegativity Difference You can also use our tool as an. Why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Chlorine And Carbon Electronegativity Difference.
From www.chemtopper.com
Video quiz on electronegativity part 4 on polarity in organic molecules due to change in Chlorine And Carbon Electronegativity Difference Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. What happens if two atoms of equal electronegativity bond together?. Chlorine And Carbon Electronegativity Difference.
From www.slideshare.net
Electronegativity part two Chlorine And Carbon Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. You can also use our tool as an. The electronegativity calculator allows you to calculate the type of bond. Chlorine And Carbon Electronegativity Difference.
From slideplayer.com
CHEMICAL BONDING. ppt download Chlorine And Carbon Electronegativity Difference Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. What happens if two atoms of equal electronegativity bond together? The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. You can also use our tool as an. Why. Chlorine And Carbon Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Chlorine And Carbon Electronegativity Difference Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Why does electronegativity increase across a period? 119 rows. Chlorine And Carbon Electronegativity Difference.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Chlorine And Carbon Electronegativity Difference Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Why does electronegativity increase across a period? The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding.. Chlorine And Carbon Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. What happens if two atoms of equal electronegativity bond together? Consider sodium at the beginning of period 3 and chlorine. Chlorine And Carbon Electronegativity Difference.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.5.1 Electronegativity翰林国际教育 Chlorine And Carbon Electronegativity Difference Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). What happens if two atoms of equal electronegativity bond together? Electronegativity is used. Chlorine And Carbon Electronegativity Difference.
From www.chegg.com
Solved Compound electronegativity difference? ammonia water Chlorine And Carbon Electronegativity Difference You can also use our tool as an. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different. Chlorine And Carbon Electronegativity Difference.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Is More Electronegative Chlorine And Carbon Electronegativity Difference Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. What happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at the. Chlorine And Carbon Electronegativity Difference.
From chem.libretexts.org
8.7 Bond Polarity and Electronegativity (\(\chi\)) Chemistry LibreTexts Chlorine And Carbon Electronegativity Difference Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. 119 rows values for electronegativity run from 0 to 4. Why does electronegativity increase across a period? Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Electronegativity is used to predict whether a bond between atoms. Chlorine And Carbon Electronegativity Difference.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. What happens if two atoms of equal electronegativity bond together? The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Chlorine And Carbon Electronegativity Difference.
From mavink.com
Electronegativity Difference Chart Chlorine And Carbon Electronegativity Difference Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). You can also use our tool as an. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different. Chlorine And Carbon Electronegativity Difference.
From mungfali.com
Electronegativity Trend Explained Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. 119 rows values for electronegativity run from 0 to 4. Why does electronegativity increase across a period? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. What happens if two atoms of equal electronegativity bond. Chlorine And Carbon Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine And Carbon Electronegativity Difference Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine's larger size decreases. Chlorine And Carbon Electronegativity Difference.
From slideplayer.com
State University of New York at Brockport ppt download Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Consider sodium at the beginning of period 3. Chlorine And Carbon Electronegativity Difference.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy visual learning Chlorine And Carbon Electronegativity Difference Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Why does electronegativity increase across a period? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 119 rows values for electronegativity run from 0 to 4. Chlorine's larger size decreases the effectiveness of orbital overlap in. Chlorine And Carbon Electronegativity Difference.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Chlorine And Carbon Electronegativity Difference 119 rows values for electronegativity run from 0 to 4. You can also use our tool as an. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Calculate the electronegativity. Chlorine And Carbon Electronegativity Difference.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Chlorine And Carbon Electronegativity Difference Why does electronegativity increase across a period? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. What happens if two atoms of equal electronegativity bond together? The electronegativity calculator allows you to calculate the type of bond formed between different elements using. Chlorine And Carbon Electronegativity Difference.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Chlorine And Carbon Electronegativity Difference Why does electronegativity increase across a period? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 119 rows values for electronegativity run from 0 to 4. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Chlorine's larger size decreases the effectiveness of orbital overlap in. Chlorine And Carbon Electronegativity Difference.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar/nonpolar classification Chlorine And Carbon Electronegativity Difference Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. You can also use our tool as an. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and.. Chlorine And Carbon Electronegativity Difference.
From www.vedantu.com
Chemistry Chapter 4 Chemical Bonding Class 11 Notes Chlorine And Carbon Electronegativity Difference Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. What happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 119 rows values for electronegativity run from 0 to 4. The electronegativity calculator allows you to calculate the type of bond formed between. Chlorine And Carbon Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine And Carbon Electronegativity Difference Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). 119 rows values for electronegativity run from 0 to 4. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. What happens if two atoms of equal electronegativity bond together? You. Chlorine And Carbon Electronegativity Difference.
From www.slideserve.com
PPT Properties of Carbon Element PowerPoint Presentation, free download ID4693595 Chlorine And Carbon Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. 119 rows values for electronegativity run from 0 to 4. What happens if two atoms of equal electronegativity bond together? Calculate. Chlorine And Carbon Electronegativity Difference.
From www.youtube.com
Electronegativity Difference Example YouTube Chlorine And Carbon Electronegativity Difference You can also use our tool as an. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. What happens if two atoms of equal electronegativity bond together? The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine's larger size decreases the effectiveness of. Chlorine And Carbon Electronegativity Difference.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Chlorine And Carbon Electronegativity Difference 119 rows values for electronegativity run from 0 to 4. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. What happens if. Chlorine And Carbon Electronegativity Difference.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Chlorine And Carbon Electronegativity Difference What happens if two atoms of equal electronegativity bond together? Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you to calculate the type of bond formed between different elements. Chlorine And Carbon Electronegativity Difference.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustration of elektronegatief, laag Chlorine And Carbon Electronegativity Difference Why does electronegativity increase across a period? 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. What happens if two atoms of equal. Chlorine And Carbon Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to determine the bond. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding.. Chlorine And Carbon Electronegativity Difference.
From mavink.com
Electronegativity Of All Elements Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. 119 rows values for electronegativity run from 0 to 4. What happens if two atoms of equal electronegativity bond together? You can also use our tool as an. The two chlorine atoms share the pair of electrons in the single covalent. Chlorine And Carbon Electronegativity Difference.
From slideplayer.com
Chemical Bonding. ppt download Chlorine And Carbon Electronegativity Difference Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. You can also use our tool as an. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. The electronegativity calculator allows you. Chlorine And Carbon Electronegativity Difference.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Chlorine And Carbon Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Why does electronegativity increase across a period? The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. What happens. Chlorine And Carbon Electronegativity Difference.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Chlorine And Carbon Electronegativity Difference Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. What happens if two atoms of equal electronegativity bond together? The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Calculate the electronegativity difference (δen) and average (en) of the two electronegativities, and use the table below to. Chlorine And Carbon Electronegativity Difference.
From www.pinterest.com
Electronegativity Definition and Trend Chlorine And Carbon Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Electronegativity is used to predict whether a bond between. Chlorine And Carbon Electronegativity Difference.