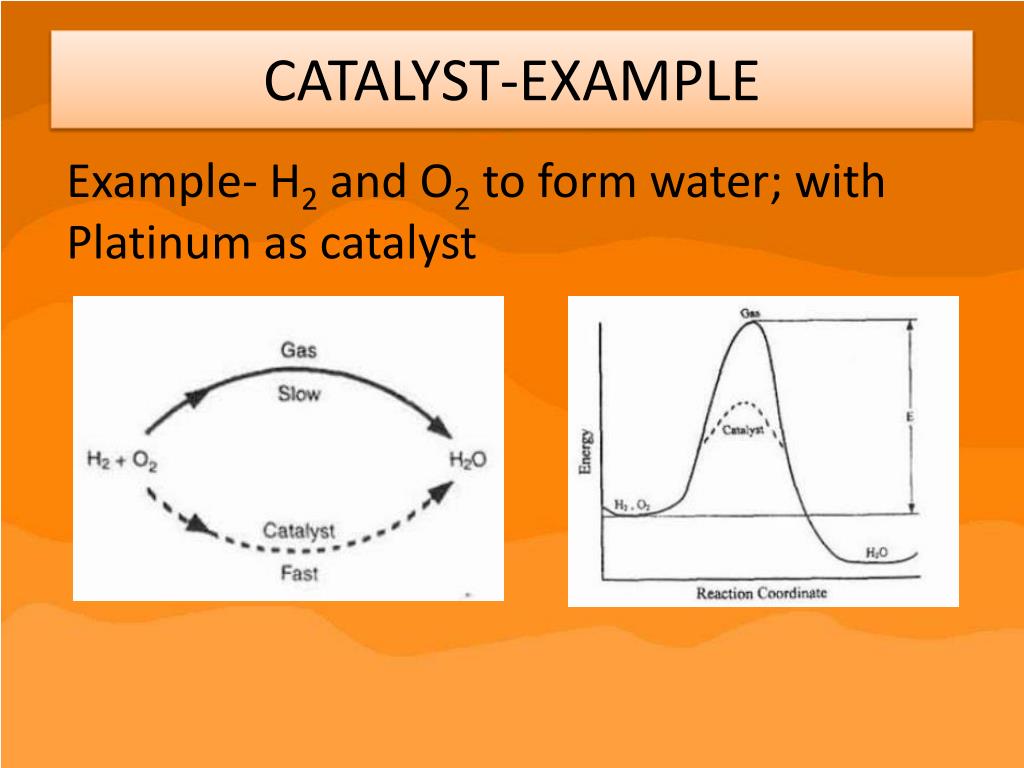
Catalyst Use Example . Enzymes are naturally occurring catalysts responsible for many. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. A very small amount of catalyst is required. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. The single biggest user of. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It’s important to remember that the catalyst.
from www.slideserve.com
Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. A very small amount of catalyst is required. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. The single biggest user of. It’s important to remember that the catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Enzymes are naturally occurring catalysts responsible for many.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalyst Use Example A very small amount of catalyst is required. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. It’s important to remember that the catalyst. A catalyst is some material that speeds up chemical reactions. A very small amount of catalyst is required. The single biggest user of. Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Use Example These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. It’s important to remember that the catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or. Catalyst Use Example.
From 2012books.lardbucket.org
Catalysis Catalyst Use Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. It’s important to remember that the catalyst. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalysis is a process of increasing the. Catalyst Use Example.
From www.slideshare.net
Biology 2.4 Catalyst Use Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is some material that speeds up chemical reactions. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. A very. Catalyst Use Example.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Use Example Enzymes are naturally occurring catalysts responsible for many. The single biggest user of. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. With a helping hand from a catalyst, molecules. Catalyst Use Example.
From essentialchemicalindustry.com
Catalysis in industry Catalyst Use Example The single biggest user of. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. It’s important to remember that the catalyst. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysis. Catalyst Use Example.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Use Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Enzymes are naturally occurring catalysts responsible for many. A catalyst is some material that speeds up chemical reactions. The single biggest. Catalyst Use Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalyst Use Example These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. Catalyst Use Example.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Use Example These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. It’s important to remember that the catalyst. Catalysis is a process of increasing. Catalyst Use Example.
From www.researchgate.net
1. Examples of catalysts used in the (a) basecatalyzed and (b Catalyst Use Example A very small amount of catalyst is required. A catalyst is some material that speeds up chemical reactions. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. The single biggest user of. With a helping hand from a catalyst, molecules that might take years. Catalyst Use Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. These examples of catalysts show how one action or one person, both in chemistry and. Catalyst Use Example.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Use Example Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyst is some material that speeds up chemical reactions. A very small amount of catalyst is required. With a helping hand from a catalyst, molecules that might take years to interact can now do so in. Catalyst Use Example.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Use Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. With a helping hand from a catalyst, molecules that. Catalyst Use Example.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Use Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. With a helping hand from a catalyst, molecules that might take years to interact. Catalyst Use Example.
From www.startswithy.com
CATALYST in a Sentence Examples 21 Ways to Use Catalyst Catalyst Use Example A very small amount of catalyst is required. It’s important to remember that the catalyst. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure. Catalyst Use Example.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Use Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A very small amount of catalyst is required. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to. Catalyst Use Example.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Use Example It’s important to remember that the catalyst. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Enzymes are naturally occurring catalysts responsible for many. A very small amount of catalyst is required. These examples of catalysts show how one. Catalyst Use Example.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Use Example A catalyst is some material that speeds up chemical reactions. A very small amount of catalyst is required. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take. Catalyst Use Example.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Use Example A very small amount of catalyst is required. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning. Catalyst Use Example.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Use Example The single biggest user of. A very small amount of catalyst is required. A catalyst is some material that speeds up chemical reactions. It’s important to remember that the catalyst. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. A catalyst is a substance that speeds up. Catalyst Use Example.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Use Example The single biggest user of. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. These examples of catalysts show how one action or one person, both in chemistry and in life, can be. Catalyst Use Example.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Use Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. These examples of catalysts show how one action or one person, both in chemistry and. Catalyst Use Example.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Use Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is some material that speeds up chemical reactions. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalysis is a process of. Catalyst Use Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID4514171 Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a. Catalyst Use Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Use Example The single biggest user of. A catalyst is some material that speeds up chemical reactions. A very small amount of catalyst is required. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Catalyst Use Example.
From www.catalystseurope.org
How are catalysts used? Catalyst Use Example The single biggest user of. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A very small amount of catalyst is required. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger. Catalyst Use Example.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalyst Use Example The single biggest user of. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. A catalyst is some material that speeds up chemical reactions. It’s important to remember that the catalyst. A very small amount of catalyst is required. Catalysis is a process of increasing the rate. Catalyst Use Example.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Catalyst Use Example A very small amount of catalyst is required. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. The single biggest user of. A. Catalyst Use Example.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Use Example Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts are used in the production of over 20% of all industrial products and. Catalyst Use Example.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. The single biggest user of. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. These examples of catalysts show how one action or one person, both in. Catalyst Use Example.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysis is a process of increasing the rate of a chemical reaction by adding a. Catalyst Use Example.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, or. Catalyst Use Example.
From www.slideshare.net
Catalyst Catalyst Use Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a. Catalyst Use Example.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Use Example It’s important to remember that the catalyst. The single biggest user of. These examples of catalysts show how one action or one person, both in chemistry and in life, can be the beginning of a larger change. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst,. Catalyst Use Example.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Use Example A very small amount of catalyst is required. Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. The single biggest user of. It’s important to remember that the catalyst. With a helping hand from a catalyst, molecules that might take years to interact can now do so. Catalyst Use Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalyst Use Example Catalysts are used in the production of over 20% of all industrial products and 90% of all chemicals and materials produced worldwide. A very small amount of catalyst is required. The single biggest user of. Enzymes are naturally occurring catalysts responsible for many. It’s important to remember that the catalyst. With a helping hand from a catalyst, molecules that might. Catalyst Use Example.