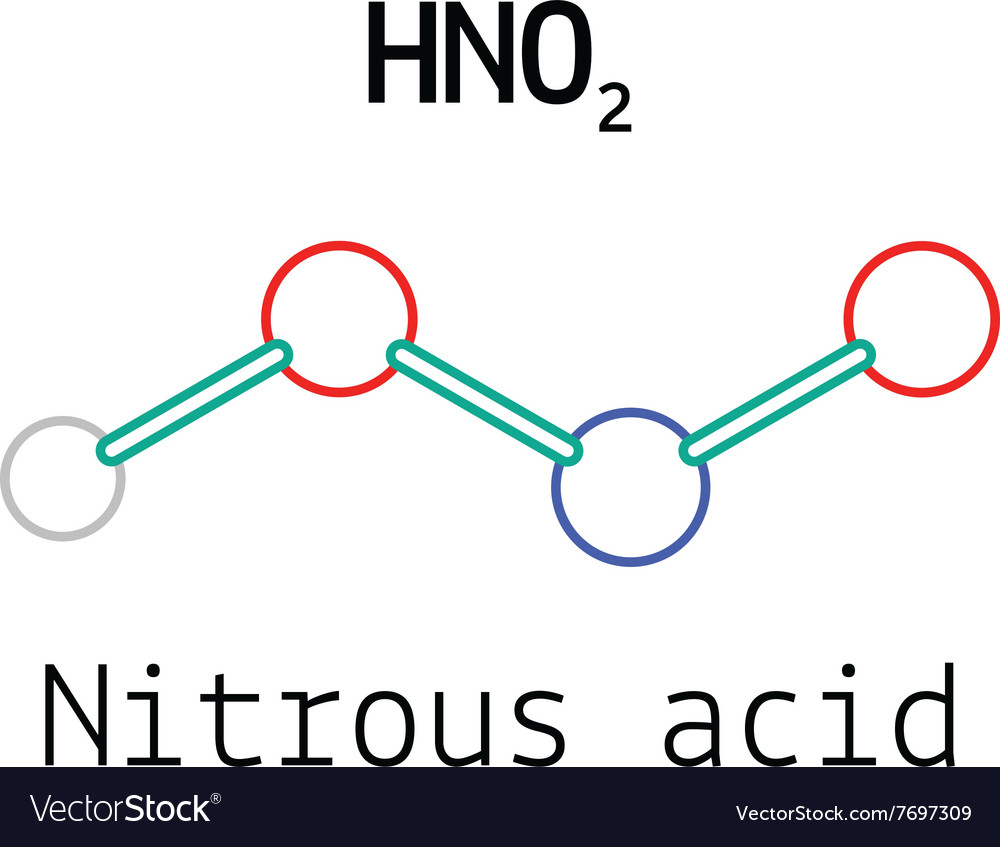
Formula For Nitrous Acid . One method is when nitrous. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. It is obtained by acidification of nitrite salt with mineral acid or. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Nitrous acid is a weak monobasic acid with the chemical formula hno2.
from www.animalia-life.club
The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Learn how to prepare, identify and use nitrous acid, and its. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. One method is when nitrous. It is obtained by acidification of nitrite salt with mineral acid or. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid is a weak monobasic acid with the chemical formula hno2.
Nitrous Acid Structure
Formula For Nitrous Acid Learn how to prepare, identify and use nitrous acid, and its. It is obtained by acidification of nitrite salt with mineral acid or. Nitrous acid is a weak monobasic acid with the chemical formula hno2. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. One method is when nitrous.
From www.cgtrader.com
Nitric acid 3D Model HNO3 free 3D model CGTrader Formula For Nitrous Acid The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts. Formula For Nitrous Acid.
From byjus.com
methylamine on treatment with nitrous acid gives 1.methanol 2.methyl Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. It is obtained by acidification of nitrite salt with mineral acid or. One method is when nitrous. The nitrous. Formula For Nitrous Acid.
From www.dreamstime.com
Nitrous Acid Stock Illustrations 56 Nitrous Acid Stock Illustrations Formula For Nitrous Acid Nitrous acid is a weak monobasic acid with the chemical formula hno2. One method is when nitrous. Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons. Formula For Nitrous Acid.
From www.chemicals.co.uk
What Is Nitric Acid? The Chemistry Blog Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. It is obtained. Formula For Nitrous Acid.
From brainly.in
Chemical formula of nitric acid by criss cross method Brainly.in Formula For Nitrous Acid One method is when nitrous. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong. Formula For Nitrous Acid.
From materikimia.com
10 Contoh Ikatan Kovalen Rangkap 2 Materi Kimia Formula For Nitrous Acid Learn how to prepare, identify and use nitrous acid, and its. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. One method is when nitrous. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid (hno. Formula For Nitrous Acid.
From edu.rsc.org
Nitric acid Resource RSC Education Formula For Nitrous Acid Nitrous acid is a weak monobasic acid with the chemical formula hno2. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. One method is when nitrous. It is obtained by acidification of. Formula For Nitrous Acid.
From www.youtube.com
Chemical Properties of Nitric Acid YouTube Formula For Nitrous Acid It is obtained by acidification of nitrite salt with mineral acid or. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Nitrous acid (hno 2) is a weak acid. Formula For Nitrous Acid.
From ar.inspiredpencil.com
Nitrous Acid Formula For Nitrous Acid The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. Nitrous acid is a weak monobasic acid with the chemical formula hno2. It is obtained by acidification of nitrite. Formula For Nitrous Acid.
From www.dreamstime.com
Nitrous Acid, Molecular Formula Stock Vector Illustration of oxide Formula For Nitrous Acid Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Learn how to prepare, identify and use nitrous acid, and its. One method is when nitrous. Nitrous acid (hno 2) is a weak. Formula For Nitrous Acid.
From www.dreamstime.com
Nitrous Acid Molecular Structure Isolated on White Stock Illustration Formula For Nitrous Acid The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Learn how to prepare, identify and use nitrous acid, and its. It is obtained by acidification of nitrite salt with mineral acid or.. Formula For Nitrous Acid.
From www.laboratorynotes.com
Nitric Acid Molecular Weight Calculation Laboratory Notes Formula For Nitrous Acid One method is when nitrous. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. It is obtained by acidification of nitrite. Formula For Nitrous Acid.
From www.youtube.com
How to write the formula for Nitrous acid (HNO2) YouTube Formula For Nitrous Acid It is obtained by acidification of nitrite salt with mineral acid or. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Nitrous acid is a weak monobasic acid with the chemical formula. Formula For Nitrous Acid.
From www.reddit.com
FoRmUlA fOr NiTrIc AcId Is NO r/woooosh Formula For Nitrous Acid Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. It is obtained by acidification of nitrite salt with mineral acid or. Nitrous acid is a weak monobasic acid with the chemical formula hno2. One method is when nitrous. Learn how to prepare, identify and use nitrous acid, and its. The lewis structure. Formula For Nitrous Acid.
From www.vectorstock.com
Nitric acid formula Royalty Free Vector Image VectorStock Formula For Nitrous Acid Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Learn how. Formula For Nitrous Acid.
From www.differencebetween.com
Difference Between Nitric Acid and Nitrous Acid Compare the Formula For Nitrous Acid The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. It is obtained by acidification of nitrite salt with mineral acid or. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Out of these 9 electron pairs, there. Formula For Nitrous Acid.
From www.numerade.com
SOLVED The formula for nitrous acid is HNOz When starting to draw the Formula For Nitrous Acid The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. It is obtained by acidification of nitrite salt with mineral acid or. Nitrous acid is a weak monobasic acid with. Formula For Nitrous Acid.
From www.numerade.com
SOLVED Nitrous acid (HNO2) disproportionates in acidic solutionto Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron. Formula For Nitrous Acid.
From www.animalia-life.club
Nitrous Acid Structure Formula For Nitrous Acid Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. It is obtained by acidification of nitrite salt with mineral acid or. Nitrous acid is a weak monobasic acid with the chemical formula. Formula For Nitrous Acid.
From techalim27.blogspot.com
Nitric Acid\\ Preparation\\Uses Formula For Nitrous Acid Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. It is obtained by acidification of nitrite salt with mineral acid or. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent.. Formula For Nitrous Acid.
From www.animalia-life.club
Nitrous Acid Structure Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. It is obtained by acidification of nitrite salt with mineral acid or. Out of these 9 electron pairs, there are. Formula For Nitrous Acid.
From www.iflscience.com
Say Hello To Nitric Acid, A Corrosive Formula That's Rained On Your Formula For Nitrous Acid It is obtained by acidification of nitrite salt with mineral acid or. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of. Formula For Nitrous Acid.
From www.dreamstime.com
Nitrous Acid Stock Illustrations 56 Nitrous Acid Stock Illustrations Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron. Formula For Nitrous Acid.
From www.chegg.com
Solved The chemical formula for nitric acid is HNO 3 Formula For Nitrous Acid The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. One method is when nitrous. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid (hno 2) is a weak. Formula For Nitrous Acid.
From www.youtube.com
Nitrous acid Meaning YouTube Formula For Nitrous Acid Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Nitric acid, hno. Formula For Nitrous Acid.
From alivechemicals.blogspot.com
Nitric Acid Formula For Nitrous Acid Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how. Formula For Nitrous Acid.
From www.myxxgirl.com
Nitrate Chemical Formula My XXX Hot Girl Formula For Nitrous Acid One method is when nitrous. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Learn how to. Formula For Nitrous Acid.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Nitric acid (fuming nitric Formula For Nitrous Acid Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid is a weak monobasic acid with the chemical formula hno2. Learn how to prepare, identify and use nitrous acid, and its.. Formula For Nitrous Acid.
From www.numerade.com
SOLVED The formula for nitrous acid is HNO2. When starting to draw the Formula For Nitrous Acid Learn how to prepare, identify and use nitrous acid, and its. Nitrous acid is a weak monobasic acid with the chemical formula hno2. It is obtained by acidification of nitrite salt with mineral acid or. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Out of these 9 electron. Formula For Nitrous Acid.
From www.fishersci.pt
Nitric Acid 6870 d=1.42, Certified AR, for Analysis, Fisher Chemical Formula For Nitrous Acid It is obtained by acidification of nitrite salt with mineral acid or. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. Nitrous acid is a weak monobasic acid. Formula For Nitrous Acid.
From www.dreamstime.com
Nitrous Acid Molecular Structure Isolated on White Stock Illustration Formula For Nitrous Acid Nitrous acid is a weak monobasic acid with the chemical formula hno2. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. One method is when nitrous. It is obtained by acidification. Formula For Nitrous Acid.
From www.showme.com
Formula for nitrous acid Science, Chemistry ShowMe Formula For Nitrous Acid Nitrous acid (hno 2) is a weak acid that exists in solution or as nitrite salts only. It is obtained by acidification of nitrite salt with mineral acid or. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. Learn how to prepare, identify and use nitrous acid, and its. Nitric acid, hno. Formula For Nitrous Acid.
From www.lookfordiagnosis.com
Nitrous acid Formula For Nitrous Acid The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. One method is when nitrous. Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a. Formula For Nitrous Acid.
From keywordsuggest.org
Image Gallery nitrous acid Formula For Nitrous Acid Learn how to prepare, identify and use nitrous acid, and its. Out of these 9 electron pairs, there are 4 bond pairs and 5 lone pairs of electrons. The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. It is obtained by acidification of nitrite salt with mineral acid or.. Formula For Nitrous Acid.
From www.numerade.com
SOLVED Write the formula for each of the acids listed below 1. Nitric Formula For Nitrous Acid The nitrous acid chemical formula is hno 2, and there are two primary ways in which nitrous acid can decompose. The lewis structure for nitrous acid (hno 2) displays a total of 18 valence electrons i.e., 18/2 = 9 electron pairs. Learn how to prepare, identify and use nitrous acid, and its. Out of these 9 electron pairs, there are. Formula For Nitrous Acid.