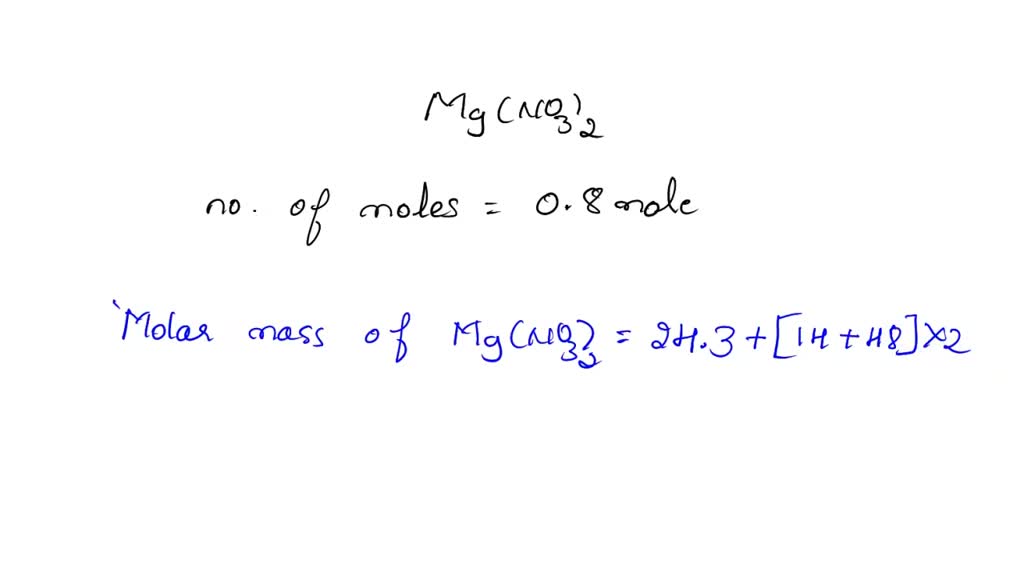What Is The Oxidation Number Of N In Mg(No3)2 . What is the oxidation state of oxygen in mg(no 3 ) 02 ? Enhanced with ai, our expert help has broken down your. Enter the formula of a chemical compound to find the oxidation number of each element. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. A net ionic charge can be specified at the end of the. The oxidation number of mg in mg(no3)2 is +2. Standard oxidation numbers of some elements are known. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation number of n in mg(no3)2 is +5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go!
from www.vrogue.co
Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. A net ionic charge can be specified at the end of the. The oxidation number of n in mg(no3)2 is +5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Standard oxidation numbers of some elements are known. What is the oxidation state of oxygen in mg(no 3 ) 02 ? The oxidation number of mg in mg(no3)2 is +2. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Enhanced with ai, our expert help has broken down your. Enter the formula of a chemical compound to find the oxidation number of each element.
Magnesium Sulfate Salt Molar Mass Physical Chemical P vrogue.co
What Is The Oxidation Number Of N In Mg(No3)2 The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. A net ionic charge can be specified at the end of the. Enhanced with ai, our expert help has broken down your. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation number of mg in mg(no3)2 is +2. Standard oxidation numbers of some elements are known. Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! What is the oxidation state of oxygen in mg(no 3 ) 02 ? The oxidation number of n in mg(no3)2 is +5. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5.
From www.slideserve.com
PPT Hydrogen Gas from the Reaction of Magnesium Metal With Acid What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. Enhanced with ai, our expert help has broken down your. Standard oxidation numbers of some elements are known. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x. What Is The Oxidation Number Of N In Mg(No3)2.
From w20.b2m.cz
Zn Mg No3 2 EDUCA What Is The Oxidation Number Of N In Mg(No3)2 A net ionic charge can be specified at the end of the. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Enhanced with ai, our expert help has broken down your. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the. What Is The Oxidation Number Of N In Mg(No3)2.
From www.hotzxgirl.com
Chemistry Oxidation Numbers Hot Sex Picture What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. Standard oxidation numbers of some elements are known. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. The oxidation number of n in mg(no3)2 is +5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! A net ionic charge can. What Is The Oxidation Number Of N In Mg(No3)2.
From www.bartleby.com
Answered 1311 Practice Questions Oxidation and… bartleby What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Standard oxidation numbers of some elements are known. A net ionic charge can be specified at the end of the. The oxidation number of n in mg(no3)2 is +5. Enhanced with ai,. What Is The Oxidation Number Of N In Mg(No3)2.
From treatybottle13.pythonanywhere.com
Smart Galvanic Cell Khan Academy Potential Energy Calculator Omni What Is The Oxidation Number Of N In Mg(No3)2 Standard oxidation numbers of some elements are known. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of n in mg(no3)2 is. What Is The Oxidation Number Of N In Mg(No3)2.
From www.toppr.com
Calculate the oxidation number of sulphur, chromium and nitrogen in What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. What is the oxidation state of oxygen. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
How to find the Oxidation Number for Fe in Fe(NO3)2 YouTube What Is The Oxidation Number Of N In Mg(No3)2 What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of n in mg(no3)2 is +5. The oxidation number of mg in mg(no3)2 is +2. A net ionic charge can be specified at the end of the. What. What Is The Oxidation Number Of N In Mg(No3)2.
From www.numerade.com
SOLVEDList the different nitrogen oxides. What is the oxidation number What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Enter the formula of a chemical compound to find the oxidation number of each element. Enhanced with ai, our expert help has broken down your. What is the oxidation state of oxygen. What Is The Oxidation Number Of N In Mg(No3)2.
From www.gutefrage.net
Eine Frage zur Berechnung von Oxidationszahlen? (Schule, Chemie, Alkohol) What Is The Oxidation Number Of N In Mg(No3)2 The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. The oxidation number of n in mg(no3)2 is +5. The oxidation number of mg in mg(no3)2 is +2. Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation state of oxygen in mg(no 3 ) 02. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
How to find the Oxidation Number for N in Ba(NO3)2 (Barium nitrate What Is The Oxidation Number Of N In Mg(No3)2 What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! The oxidation number of mg in mg(no3)2 is +2. Enhanced with ai, our expert help has broken down your. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. A net ionic charge can be specified at the end of the.. What Is The Oxidation Number Of N In Mg(No3)2.
From www.toppr.com
(i) Balance the following equations by oxidation number method (1) Cu What Is The Oxidation Number Of N In Mg(No3)2 A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Enhanced with ai, our expert help. What Is The Oxidation Number Of N In Mg(No3)2.
From uirunisaza.web.fc2.com
Determine the oxidation number of each element in the following What Is The Oxidation Number Of N In Mg(No3)2 What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Therefore, the oxidation. What Is The Oxidation Number Of N In Mg(No3)2.
From www.chegg.com
Solved 2, Ca + HNO3 → Ca(NO3)2 + H2 Assign oxidation numbers What Is The Oxidation Number Of N In Mg(No3)2 What is the oxidation state of oxygen in mg(no 3 ) 02 ? What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Standard oxidation numbers of some elements are known. The oxidation number of n in mg(no3)2 is +5. A net ionic charge can be specified at the end of the. Enter the formula of. What Is The Oxidation Number Of N In Mg(No3)2.
From www.numerade.com
SOLVEDNitrogen dioxide can be formed from the reaction of nitric oxide What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. What is the oxidation state of oxygen in mg(no 3 ) 02 ? The oxidation number of n in mg(no3)2 is +5. The oxidation number of each atom can be calculated by subtracting the sum of. What Is The Oxidation Number Of N In Mg(No3)2.
From www.nagwa.com
Question Video Identifying the Oxidation Number of Nitrogen in Nitric What Is The Oxidation Number Of N In Mg(No3)2 Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Standard oxidation numbers of some elements are known. The average oxidation state of the 2 n atoms. What Is The Oxidation Number Of N In Mg(No3)2.
From www.tutoroot.com
What is Nitrogen Cycle? Diagram, Stages, Importance Tutoroot What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of n in mg(no3)2 is +5. Enhanced with ai, our expert help has broken down your. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. What is the oxidation state of oxygen in mg(no 3 ) 02. What Is The Oxidation Number Of N In Mg(No3)2.
From www.numerade.com
SOLVED what is the oxidation number of Pb in Pb(NO3)2 What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. Enhanced with ai, our expert help has broken down your. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. Enter the formula of a chemical compound to find the oxidation number of each element. Standard oxidation numbers of some elements are known. The oxidation number. What Is The Oxidation Number Of N In Mg(No3)2.
From katelynnkruwblake.blogspot.com
How Do I Know Which Oxidation State to Use KatelynnkruwBlake What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! The oxidation number of n in mg(no3)2 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
How to Write the Name for Mg(NO3)2 YouTube What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. The oxidation number of n in mg(no3)2 is +5. What is the oxidation state of oxygen in mg(no 3 ) 02 ? Therefore, the oxidation number of nitrogen in m g (n o x 3) x. What Is The Oxidation Number Of N In Mg(No3)2.
From brainly.in
What is oxidation number of nitrogen in ammonium nitrate? Brainly.in What Is The Oxidation Number Of N In Mg(No3)2 Standard oxidation numbers of some elements are known. The oxidation number of mg in mg(no3)2 is +2. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. What is the oxidation state of oxygen in mg(no 3 ) 02 ? Enter. What Is The Oxidation Number Of N In Mg(No3)2.
From www.vrogue.co
Magnesium Sulfate Salt Molar Mass Physical Chemical P vrogue.co What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of mg in mg(no3)2 is +2. A net ionic charge can be specified at the end of the. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. Enhanced with ai, our expert help has broken down your.. What Is The Oxidation Number Of N In Mg(No3)2.
From www.numerade.com
SOLVED 11) From the following equation show the oxidation numbers for What Is The Oxidation Number Of N In Mg(No3)2 A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation state of oxygen in mg(no 3 ) 02 ? The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains. What Is The Oxidation Number Of N In Mg(No3)2.
From askfilo.com
What is the value of oxidation number of nitrogen atoms in NH4 NO3 −.. What Is The Oxidation Number Of N In Mg(No3)2 Enhanced with ai, our expert help has broken down your. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. What is the oxidation state of oxygen in mg(no 3 ) 02 ? Enter the formula of a chemical compound to find the oxidation number of each element. Standard oxidation numbers of some elements are. What Is The Oxidation Number Of N In Mg(No3)2.
From ar.inspiredpencil.com
Oxidation Number What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. Enhanced with ai, our expert help has broken down your. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3. What Is The Oxidation Number Of N In Mg(No3)2.
From www.quora.com
What are the oxidation numbers of C and N in HNC? Quora What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. Standard oxidation numbers of some elements are known. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. The oxidation number of each atom can be. What Is The Oxidation Number Of N In Mg(No3)2.
From www.sliderbase.com
Oxidation Numbers and names What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! The oxidation number of n in mg(no3)2 is +5. Enhanced with ai, our expert help has. What Is The Oxidation Number Of N In Mg(No3)2.
From www.nagwa.com
Question Video Deducing the Oxidation State of Chlorine in the What Is The Oxidation Number Of N In Mg(No3)2 Standard oxidation numbers of some elements are known. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number. What Is The Oxidation Number Of N In Mg(No3)2.
From ar.inspiredpencil.com
Periodic Table Of Elements With Oxidation Numbers What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. Standard oxidation numbers of some elements are known. The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds. What Is The Oxidation Number Of N In Mg(No3)2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Mg(No3)2 What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation number of n in mg(no3)2 is +5. The oxidation number of mg in mg(no3)2 is +2.. What Is The Oxidation Number Of N In Mg(No3)2.
From xaydungso.vn
Cách vẽ no3 lewis structure đúng chuẩn và nhanh nhất What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! The average oxidation state of the 2 n atoms in mg(no 3) 02 is +5. What is the oxidation state of oxygen in mg(no 3 ) 02 ? Therefore, the oxidation. What Is The Oxidation Number Of N In Mg(No3)2.
From brainly.in
What is oxidation number of N in Cu(NO3)2 ? Plz show me how did you What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. A net ionic charge can be specified at the end of the. Standard oxidation numbers of some. What Is The Oxidation Number Of N In Mg(No3)2.
From www.animalia-life.club
H2po4 Lewis Structure What Is The Oxidation Number Of N In Mg(No3)2 Enter the formula of a chemical compound to find the oxidation number of each element. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. A net ionic charge can be specified at the end of the. What is the oxidation. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
Redox balance Ag +NO3 =Ag+ + NO Half reaction method Ion What Is The Oxidation Number Of N In Mg(No3)2 Standard oxidation numbers of some elements are known. The oxidation number of mg in mg(no3)2 is +2. What is the oxidation state of nitrogen in mg(no3)2 your solution’s ready to go! Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5.. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
How to find the Oxidation Number for Mn in Mn(NO3)2 YouTube What Is The Oxidation Number Of N In Mg(No3)2 The oxidation number of n in mg(no3)2 is +5. Therefore, the oxidation number of nitrogen in m g (n o x 3) x 2 \ce{mg(no3)2} mg (no x 3 ) x 2 is + 5 +5 + 5. Enhanced with ai, our expert help has broken down your. Standard oxidation numbers of some elements are known. The oxidation number of. What Is The Oxidation Number Of N In Mg(No3)2.
From www.youtube.com
How to find the Oxidation Number for N in Mg(NO3)2 (Magnesium nitrate What Is The Oxidation Number Of N In Mg(No3)2 A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Enhanced with ai, our expert help has broken down your. What is the oxidation state of oxygen in mg(no 3. What Is The Oxidation Number Of N In Mg(No3)2.