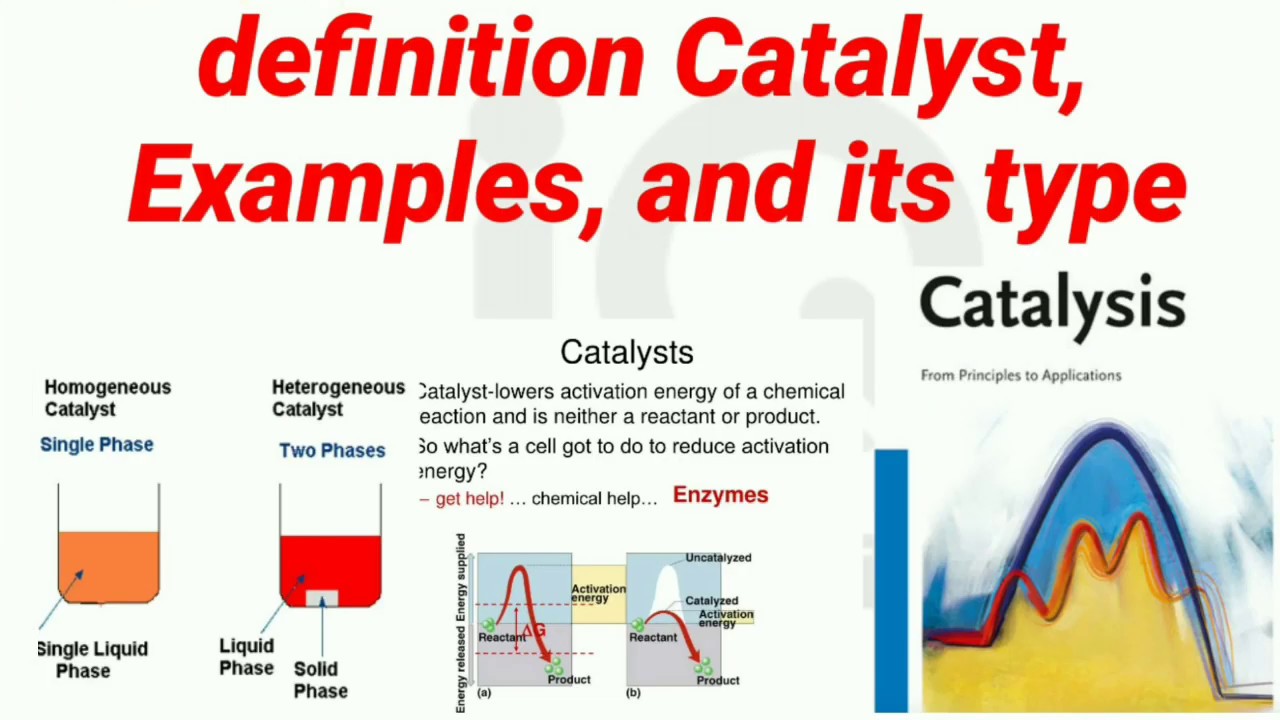
Catalyst Examples For Chemistry . Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction. Catalysts allow a reaction to. A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts participate in a chemical reaction and increase its rate.
from medicaltaste.weebly.com
A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysts allow a reaction to. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
Periodic table catalyst definition chemistry medicaltaste
Catalyst Examples For Chemistry Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal. A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysts participate in a chemical reaction and increase its rate. They provide an alternative reaction route with a lower activation. They do not appear in the reaction’s net equation and are not consumed during the reaction.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Examples For Chemistry What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance which increases the rate of a reaction without being used up itself. They do not appear in the reaction’s net equation and are. Catalyst Examples For Chemistry.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Examples For Chemistry Catalysts allow a reaction to. What are catalysts, and how do they work in terms altering the parameters of a reaction? They do not appear in the reaction’s net equation and are not consumed during the reaction. Describe the similarities and differences between the three principal. A catalyst is a substance which increases the rate of a reaction without being. Catalyst Examples For Chemistry.
From www.essentialchemicalindustry.org
Catalysis in industry Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. They provide an alternative reaction route with a lower activation. This page looks at the the. Catalyst Examples For Chemistry.
From www.slideshare.net
Catalyst Catalyst Examples For Chemistry They provide an alternative reaction route with a lower activation. Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the three principal. They do not appear in the reaction’s net equation. Catalyst Examples For Chemistry.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst Examples For Chemistry A catalyst is a substance that speeds up a chemical reaction. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance. Catalyst Examples For Chemistry.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Examples For Chemistry Describe the similarities and differences between the three principal. They provide an alternative reaction route with a lower activation. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up a chemical reaction. What are catalysts, and how do they work in terms altering the parameters of a. Catalyst Examples For Chemistry.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Examples For Chemistry They provide an alternative reaction route with a lower activation. They do not appear in the reaction’s net equation and are not consumed during the reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance which increases. Catalyst Examples For Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to. They provide an alternative reaction route with a lower activation. A catalyst is a substance that speeds up a chemical reaction. What. Catalyst Examples For Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Examples For Chemistry Catalysts allow a reaction to. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation ID676158 Catalyst Examples For Chemistry Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and. Catalyst Examples For Chemistry.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Examples For Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance which increases the rate of a reaction without being used up itself. They do not appear in the reaction’s net equation and are not consumed during the reaction.. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that speeds up a chemical reaction. They provide an alternative reaction route with a. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Examples For Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate. What are catalysts, and how do they work in terms altering the parameters of a reaction? They provide an alternative reaction route with a lower activation. Describe the similarities. Catalyst Examples For Chemistry.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Examples For Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts participate in a chemical reaction and increase its rate. What are catalysts, and how do they work in terms altering the parameters of a reaction? They provide an alternative reaction route with a lower activation.. Catalyst Examples For Chemistry.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Examples For Chemistry Catalysts allow a reaction to. Describe the similarities and differences between the three principal. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a substance which increases the rate of a reaction without being used up itself. This page looks at the the different types of catalyst (heterogeneous and homogeneous). Catalyst Examples For Chemistry.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst Examples For Chemistry Catalysts participate in a chemical reaction and increase its rate. What are catalysts, and how do they work in terms altering the parameters of a reaction? They provide an alternative reaction route with a lower activation. A catalyst is a substance that speeds up a chemical reaction. Describe the similarities and differences between the three principal. Catalysts allow a reaction. Catalyst Examples For Chemistry.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Examples For Chemistry Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts participate in a chemical reaction and increase its rate.. Catalyst Examples For Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Examples For Chemistry Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? They provide an alternative reaction route with a lower activation. They do. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalyst Examples For Chemistry This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. Describe the similarities and differences between the three principal. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts participate in a chemical reaction and increase its rate. Catalyst, in chemistry, any substance that increases the. Catalyst Examples For Chemistry.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Examples For Chemistry They provide an alternative reaction route with a lower activation. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. They do not appear in the reaction’s net equation and are. Catalyst Examples For Chemistry.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Examples For Chemistry What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to. They provide an alternative reaction route with a lower activation. Catalysts participate in a chemical reaction and increase its rate. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and. Catalyst Examples For Chemistry.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance that speeds up a chemical reaction. Catalysts allow a reaction to. This page looks at the the different types of catalyst (heterogeneous. Catalyst Examples For Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Examples For Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the three principal. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance that speeds up a chemical reaction. A catalyst is. Catalyst Examples For Chemistry.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Examples For Chemistry Catalysts participate in a chemical reaction and increase its rate. Describe the similarities and differences between the three principal. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts allow a reaction to. A catalyst is a substance which increases the rate of a reaction. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Examples For Chemistry They provide an alternative reaction route with a lower activation. A catalyst is a substance that speeds up a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net. Catalyst Examples For Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Examples For Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal. What. Catalyst Examples For Chemistry.
From www.slideshare.net
Catalysis Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. Describe the similarities and differences between the three principal. Catalysts participate in a chemical reaction and increase its rate. What are catalysts, and how do they work in terms altering the parameters of. Catalyst Examples For Chemistry.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Examples For Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the three principal. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. They do not appear in the reaction’s net equation and are. Catalyst Examples For Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Examples For Chemistry Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up a chemical reaction. Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst Examples For Chemistry.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Examples For Chemistry They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysts participate in a chemical reaction and increase its rate. A. Catalyst Examples For Chemistry.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Examples For Chemistry What are catalysts, and how do they work in terms altering the parameters of a reaction? They do not appear in the reaction’s net equation and are not consumed during the reaction. Describe the similarities and differences between the three principal. Catalysts allow a reaction to. Catalysts participate in a chemical reaction and increase its rate. They provide an alternative. Catalyst Examples For Chemistry.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Examples For Chemistry A catalyst is a substance which increases the rate of a reaction without being used up itself. What are catalysts, and how do they work in terms altering the parameters of a reaction? They do not appear in the reaction’s net equation and are not consumed during the reaction. Describe the similarities and differences between the three principal. Catalyst, in. Catalyst Examples For Chemistry.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Examples For Chemistry What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst Examples For Chemistry.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalyst Examples For Chemistry They provide an alternative reaction route with a lower activation. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts allow a reaction to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in. Catalyst Examples For Chemistry.