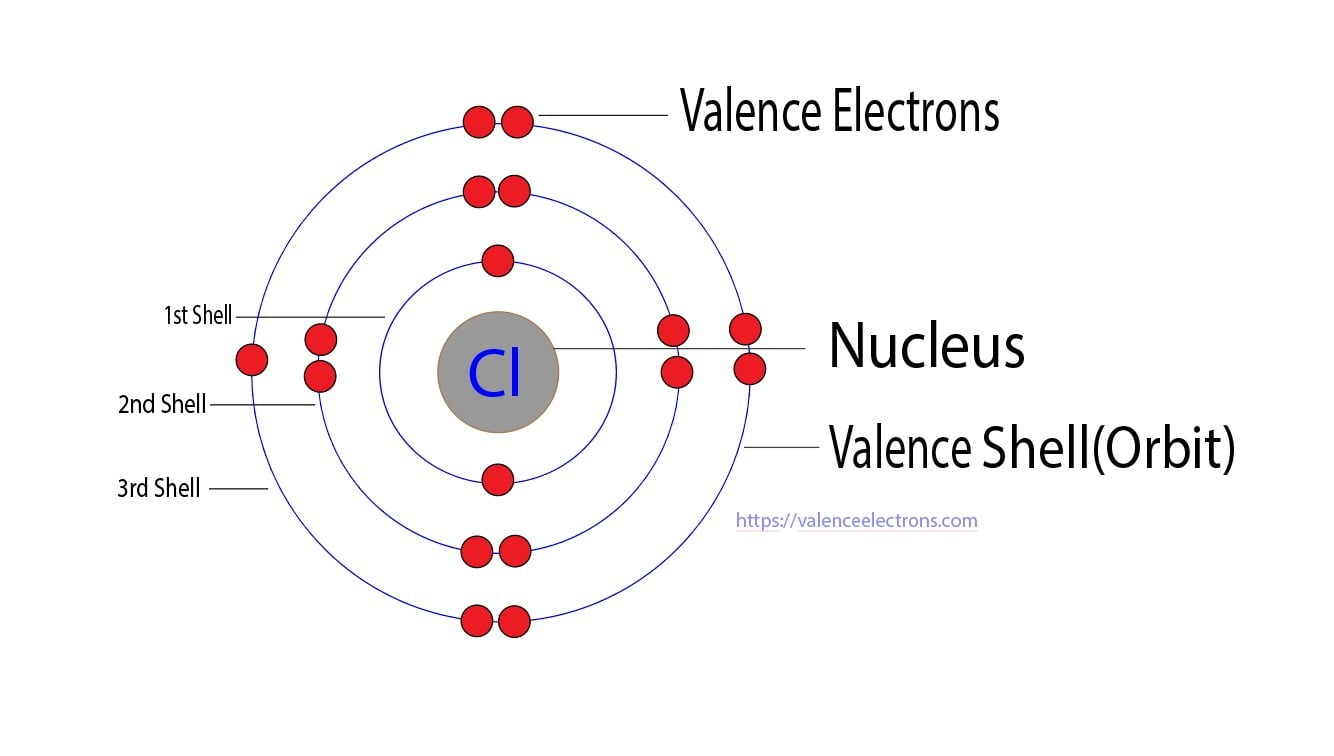
Chlorine Have Many Valence Electrons . Chlorine has 7 valence electrons. 119 rows valence electrons: This makes chlorine a cl−1. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. It needs one electron to make it stable at 8 electrons in its valence shells. How many valence electrons does chlorine have? Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Helium is the only exception for the main group elements.
from valenceelectrons.com
It needs one electron to make it stable at 8 electrons in its valence shells. Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. Helium is the only exception for the main group elements. Chlorine has 7 valence electrons. 119 rows valence electrons: Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. How many valence electrons does chlorine have?
How Many Valence Electrons Does Chlorine (Cl) Have?
Chlorine Have Many Valence Electrons Chlorine has 7 valence electrons. Chlorine has 7 valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Calcium would have two valence electrons, since it is in group iia (group 2). For example, silicon is in group iva (group 14), so each atom would have four valence electrons. You may assume the valences of the chemical elements—the number of electrons with which. This makes chlorine a cl−1. 93 rows updated on may 19, 2024. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is in group viia (group 17), so it would have seven valence electrons. How many valence electrons does chlorine have? Helium is the only exception for the main group elements. 119 rows valence electrons:
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Have Many Valence Electrons 93 rows updated on may 19, 2024. Chlorine is in group viia (group 17), so it would have seven valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Helium is the only exception for the main group elements. How many valence electrons does chlorine have? 119 rows valence electrons: For example, silicon. Chlorine Have Many Valence Electrons.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Have Many Valence Electrons This makes chlorine a cl−1. 119 rows valence electrons: Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. It needs one electron to make it stable at 8 electrons in its valence shells. Helium is the only exception for the main group elements. Chlorine is in group viia (group 17), so it would have. Chlorine Have Many Valence Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Have Many Valence Electrons How many valence electrons does chlorine have? Chlorine is in group viia (group 17), so it would have seven valence electrons. Chlorine has 7 valence electrons. 119 rows valence electrons: For example, silicon is in group iva (group 14), so each atom would have four valence electrons. You may assume the valences of the chemical elements—the number of electrons with. Chlorine Have Many Valence Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl Chlorine Have Many Valence Electrons Chlorine has 7 valence electrons. How many valence electrons does chlorine have? This makes chlorine a cl−1. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is in group viia (group 17), so it would have seven valence. Chlorine Have Many Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Have Many Valence Electrons Chlorine has 7 valence electrons. You may assume the valences of the chemical elements—the number of electrons with which. Chlorine is in group viia (group 17), so it would have seven valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. How many valence electrons does chlorine have? For example, silicon is in. Chlorine Have Many Valence Electrons.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Have Many Valence Electrons It needs one electron to make it stable at 8 electrons in its valence shells. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. How many valence electrons does chlorine have? Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Chlorine is in group viia (group. Chlorine Have Many Valence Electrons.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Have Many Valence Electrons 119 rows valence electrons: It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is in group viia (group 17), so it would have seven valence electrons. Helium is the only exception for the main group elements. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. This makes. Chlorine Have Many Valence Electrons.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Have Many Valence Electrons This makes chlorine a cl−1. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has 7 valence electrons. How many valence electrons does chlorine have? 93 rows updated on may 19, 2024. You may assume the valences of. Chlorine Have Many Valence Electrons.
From www.youtube.com
How many valence electrons does chlorine have?How to find the valence Chlorine Have Many Valence Electrons Chlorine has 7 valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is in group viia (group 17), so it would have seven valence electrons. How many valence electrons does chlorine have? Helium is. Chlorine Have Many Valence Electrons.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Have Many Valence Electrons Helium is the only exception for the main group elements. This makes chlorine a cl−1. How many valence electrons does chlorine have? It needs one electron to make it stable at 8 electrons in its valence shells. You may assume the valences of the chemical elements—the number of electrons with which. Because chlorine has an electronic configuration 1s 2 2s. Chlorine Have Many Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Have Many Valence Electrons Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. This makes chlorine a cl−1. You may assume the valences of the chemical elements—the number of electrons with which. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Helium is the only exception for the main group. Chlorine Have Many Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Have Many Valence Electrons Helium is the only exception for the main group elements. How many valence electrons does chlorine have? You may assume the valences of the chemical elements—the number of electrons with which. Chlorine has 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows updated on may 19, 2024. For example, silicon. Chlorine Have Many Valence Electrons.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Have Many Valence Electrons Helium is the only exception for the main group elements. 119 rows valence electrons: Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. It needs one electron to make it stable at 8 electrons in its valence shells. How many valence electrons does chlorine have? Chlorine is in group viia (group 17), so it. Chlorine Have Many Valence Electrons.
From elchoroukhost.net
Chlorine Periodic Table Electrons Elcho Table Chlorine Have Many Valence Electrons For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. How many valence electrons does chlorine have? Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since. Chlorine Have Many Valence Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Have Many Valence Electrons This makes chlorine a cl−1. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. It needs one electron to make it stable at. Chlorine Have Many Valence Electrons.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Have Many Valence Electrons This makes chlorine a cl−1. 119 rows valence electrons: Calcium would have two valence electrons, since it is in group iia (group 2). For example, silicon is in group iva (group 14), so each atom would have four valence electrons. You may assume the valences of the chemical elements—the number of electrons with which. Chlorine is in group viia (group. Chlorine Have Many Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Have Many Valence Electrons For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 119 rows valence electrons: 93 rows updated on may 19, 2024. This makes chlorine a cl−1. Calcium would have two valence electrons, since it is in group iia (group 2). It needs one electron to make it stable at 8 electrons in its. Chlorine Have Many Valence Electrons.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Have Many Valence Electrons 119 rows valence electrons: This makes chlorine a cl−1. You may assume the valences of the chemical elements—the number of electrons with which. Chlorine has 7 valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. How many valence electrons does chlorine have? Helium is the only exception for the main. Chlorine Have Many Valence Electrons.
From guide-scientific.com
¿Cuántos electrones de valencia tiene el Cloro (Cl)? Valencia de cloro. Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). You may assume the valences of the chemical elements—the number of electrons with which. Chlorine is in group viia (group 17), so it would have seven valence electrons. Helium is the only exception for the main group elements. It needs one electron to make it stable. Chlorine Have Many Valence Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Have Many Valence Electrons Chlorine is in group viia (group 17), so it would have seven valence electrons. How many valence electrons does chlorine have? For example, silicon is in group iva (group 14), so each atom would have four valence electrons. This makes chlorine a cl−1. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has. Chlorine Have Many Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Have Many Valence Electrons It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. Chlorine is in group viia (group 17), so it would have seven valence electrons. Helium is the only exception for the main group elements.. Chlorine Have Many Valence Electrons.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Have Many Valence Electrons Chlorine is in group viia (group 17), so it would have seven valence electrons. Chlorine has 7 valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). How many valence electrons does chlorine have? Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Helium is the only exception. Chlorine Have Many Valence Electrons.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Have Many Valence Electrons 119 rows valence electrons: 93 rows updated on may 19, 2024. This makes chlorine a cl−1. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. How many valence electrons does chlorine have? For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Calcium would have two valence. Chlorine Have Many Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Have Many Valence Electrons This makes chlorine a cl−1. 93 rows updated on may 19, 2024. Calcium would have two valence electrons, since it is in group iia (group 2). You may assume the valences of the chemical elements—the number of electrons with which. Helium is the only exception for the main group elements. It needs one electron to make it stable at 8. Chlorine Have Many Valence Electrons.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). This makes chlorine a cl−1. 119 rows valence electrons: Helium is the only exception for the main group elements. 93 rows updated on may 19, 2024. It needs one electron to make it stable at 8 electrons in its valence shells. How many valence electrons does. Chlorine Have Many Valence Electrons.
From www.youtube.com
How many valence electrons are in an atom of chlorine? YouTube Chlorine Have Many Valence Electrons Chlorine has 7 valence electrons. 119 rows valence electrons: Calcium would have two valence electrons, since it is in group iia (group 2). For example, silicon is in group iva (group 14), so each atom would have four valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a cl−1.. Chlorine Have Many Valence Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Have Many Valence Electrons Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. Helium is the only exception for the main group elements. This makes chlorine a cl−1. 93 rows updated on may 19, 2024. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Chlorine has 7 valence electrons. Chlorine. Chlorine Have Many Valence Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Have Many Valence Electrons 93 rows updated on may 19, 2024. How many valence electrons does chlorine have? Chlorine is in group viia (group 17), so it would have seven valence electrons. This makes chlorine a cl−1. 119 rows valence electrons: Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. You may assume. Chlorine Have Many Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 119 rows valence electrons: 93 rows updated on may 19, 2024. Chlorine is in group viia (group 17), so it would have seven valence electrons. Helium is the only. Chlorine Have Many Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Have Many Valence Electrons For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has 7 valence electrons. 119 rows valence electrons: Calcium would have two. Chlorine Have Many Valence Electrons.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Have Many Valence Electrons Because chlorine has an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p. 119 rows valence electrons: Chlorine has 7 valence electrons. You may assume the valences of the chemical elements—the number of electrons with which. This makes chlorine a cl−1. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is. Chlorine Have Many Valence Electrons.
From favpng.com
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine has 7 valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. Helium is the. Chlorine Have Many Valence Electrons.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). Helium is the only exception for the main group elements. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows updated on may 19, 2024. How many valence electrons does chlorine have? This makes chlorine a cl−1. 119 rows. Chlorine Have Many Valence Electrons.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Have Many Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). Helium is the only exception for the main group elements. How many valence electrons does chlorine have? For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 93 rows updated on may 19, 2024. You may assume the valences. Chlorine Have Many Valence Electrons.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Have Many Valence Electrons It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a cl−1. Chlorine is in group viia (group 17), so it would have seven valence electrons. 119 rows valence electrons: Helium is the only exception for the main group elements. Because chlorine has an electronic configuration 1s 2 2s 2 2p 6. Chlorine Have Many Valence Electrons.