Medical Devices In Australia . The australian tga medical device approval process explained. Medical devices include a wide range of products. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. They include things like surgical. The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The argmd provides information on the import, export and supply of medical devices within australia. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions.
from www.tga.gov.au
Medical devices include a wide range of products. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical. The australian tga medical device approval process explained. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The chart illustrates the tga approval process per device classification in australia and is available for download.
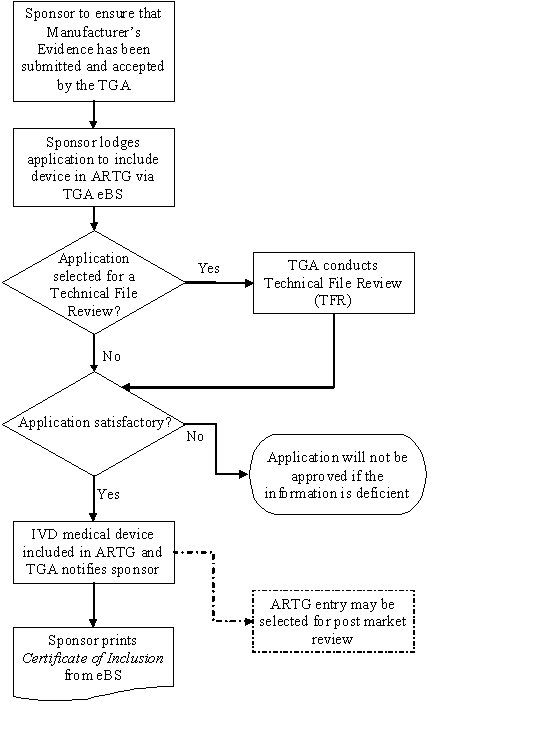
Including IVD medical devices in the ARTG Therapeutic Goods
Medical Devices In Australia The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. Medical devices include a wide range of products. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The chart illustrates the tga approval process per device classification in australia and is available for download. The australian tga medical device approval process explained. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The argmd provides information on the import, export and supply of medical devices within australia. They include things like surgical. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,.
From sleek.com
Licensing Medical Devices in Australia The Ultimate Guide Medical Devices In Australia The australian tga medical device approval process explained. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The argmd provides information on the import, export and supply of medical devices. Medical Devices In Australia.
From studylib.net
Australian regulatory guidelines for medical devices Medical Devices In Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Medical devices include a wide range of products. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024.. Medical Devices In Australia.
From www.slideshare.net
The regulation of medical devices in Australia Medical Devices In Australia The argmd provides information on the import, export and supply of medical devices within australia. Medical devices include a wide range of products. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn. Medical Devices In Australia.
From www.escomedicon.com.au
Medical Gas ESCO Medicon Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. They include things like surgical. The chart illustrates the tga approval process per device classification in australia and is available for download. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The argmd provides information on the import,. Medical Devices In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Devices In Australia The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The chart illustrates the tga approval process per device classification in australia and is available for download. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical. Provides a national system. Medical Devices In Australia.
From operonstrategist.com
Medical Device Registration in Australia (StepbyStep Guidance Medical Devices In Australia Medical devices include a wide range of products. The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The medical devices market market in australia is forecasted to achieve a revenue of. Medical Devices In Australia.
From www.tga.gov.au
Including IVD medical devices in the ARTG Therapeutic Goods Medical Devices In Australia The argmd provides information on the import, export and supply of medical devices within australia. The chart illustrates the tga approval process per device classification in australia and is available for download. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical. The medical devices information unit of the office of devices,. Medical Devices In Australia.
From medicaldevices.com.au
Broselow™ Paediatric Emergency Cart Medical Devices Australia Medical Devices In Australia Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Medical devices include a wide range of products. The australian tga medical device approval process explained. The chart illustrates the tga approval process per. Medical Devices In Australia.
From www.eclevarmedtech.com
How are software medical devices currently regulated in Australia? Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The argmd provides information on the import, export and supply of medical devices within australia. The medical devices information unit of the office of. Medical Devices In Australia.
From asiaactual.com
Australia Medical Device Market License Holding Sponsor Medical Devices In Australia The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical. The australian tga. Medical Devices In Australia.
From credevo.com
Regulations For Medical Device Approval in Australia Credevo Articles Medical Devices In Australia The chart illustrates the tga approval process per device classification in australia and is available for download. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The australian tga medical device approval process explained. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The medical devices information. Medical Devices In Australia.
From healthflume.com
10 Benefits of Medical Devices in Australia for Your Healthcare Needs Medical Devices In Australia Medical devices include a wide range of products. They include things like surgical. The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The argmd provides information on the import, export and supply of medical. Medical Devices In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Devices In Australia The australian tga medical device approval process explained. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The argmd provides information on the import, export and supply of medical devices within australia. They include things like surgical. Medical. Medical Devices In Australia.
From medicaldevices.com.au
Oakworks Imaging Tables Medical Devices Australia Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. They include things like surgical. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. Medical devices include a wide range of products. The chart illustrates the tga approval process per. Medical Devices In Australia.
From www.presentationeze.com
Australian medical device classification Rule 5—Special Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. Medical devices include a wide range of products. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The medical devices. Medical Devices In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Devices In Australia Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The australian tga medical device approval process explained. The chart illustrates the tga approval process per device classification in australia and is available for. Medical Devices In Australia.
From medicaldevices.com.au
Patient Positioning Product Range Medical Devices Australia Medical Devices In Australia The australian tga medical device approval process explained. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices include a wide range of products. The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices information unit of the office of devices, blood and. Medical Devices In Australia.
From cmsmedtech.com
medical device registration in Australia Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. They include things like surgical. The argmd provides information on the import, export and supply of medical devices within australia. The australian tga medical. Medical Devices In Australia.
From medicaldevices.com.au
Reflex Hammer Medical Devices Australia Medical Devices In Australia They include things like surgical. The australian tga medical device approval process explained. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. Medical devices include a wide range of products. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be.. Medical Devices In Australia.
From operonstrategist.com
Medical Device Registration in Australia Medical Devices In Australia The argmd provides information on the import, export and supply of medical devices within australia. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Medical devices include a wide range of products. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. They. Medical Devices In Australia.
From medicaldevices.com.au
Laryngoscopes Medical Devices Australia Medical Devices In Australia The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical. Provides a national system to control the. Medical Devices In Australia.
From medtechnique.com.au
2021, what’s next for Medical Devices in Australia? Medical Device Medical Devices In Australia Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices include a wide range of products. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The medical devices market market in. Medical Devices In Australia.
From studylib.net
The use of GMDN codes for IVD medical devices in Australia Medical Devices In Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. Medical devices include a wide range of products. They include things like surgical. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn. Medical Devices In Australia.
From medicaldevices.com.au
HiPot 150® Insulation Tester Medical Devices Australia Medical Devices In Australia They include things like surgical. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The australian tga medical device approval process explained. The chart illustrates the tga approval process per device classification in australia and is available for. Medical Devices In Australia.
From healthandcareonline.com
Essential Guide to Medical Devices and Instruments in Australia Medical Devices In Australia They include things like surgical. Medical devices include a wide range of products. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The argmd provides information on the import, export. Medical Devices In Australia.
From www.slideshare.net
TGA changes for Medical Devices in Australia Medical Devices In Australia The chart illustrates the tga approval process per device classification in australia and is available for download. The australian tga medical device approval process explained. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Medical devices include a. Medical Devices In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Devices In Australia They include things like surgical. The chart illustrates the tga approval process per device classification in australia and is available for download. The australian tga medical device approval process explained. Medical devices include a wide range of products. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The argmd provides information on the import, export and. Medical Devices In Australia.
From www.qualtechs.com
QT ANALYSIS AUSTRALIA's MEDICAL DEVICE MARKET AND REGISTRATION Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. Medical devices include a wide range of products. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The australian tga medical device approval. Medical Devices In Australia.
From medicaldevices.com.au
Surgical Headlights Medical Devices Australia Medical Devices In Australia The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The argmd provides information on the import, export and supply of medical devices within australia. The chart illustrates the tga approval process per device classification in australia and is available for download. The medical devices information unit of the office of devices,. Medical Devices In Australia.
From www.uq.edu.au
Medical technology at the hub of good health UQ News The University Medical Devices In Australia The medical devices information unit of the office of devices, blood and tissues of the therapeutic goods administration (tga) can be. The chart illustrates the tga approval process per device classification in australia and is available for download. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices can be used to diagnose,. Medical Devices In Australia.
From www.slideshare.net
Australia medical device registration and approval process EMERGO Medical Devices In Australia The chart illustrates the tga approval process per device classification in australia and is available for download. They include things like surgical. The australian tga medical device approval process explained. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The medical devices market market in australia is forecasted to achieve a. Medical Devices In Australia.
From www.regdesk.co
Australian Regulatory Guidelines for Medical Devices RegDesk Medical Devices In Australia Medical devices include a wide range of products. The argmd provides information on the import, export and supply of medical devices within australia. They include things like surgical. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The chart illustrates the tga approval process per device classification in australia and is. Medical Devices In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Devices In Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The argmd provides information on the import, export and supply of medical devices within australia. The chart illustrates the tga approval process per device classification in australia and is available for download. The australian tga medical device approval process explained. They include things like surgical. The medical. Medical Devices In Australia.
From www.artixio.com
FAQs Australia (TGA) Regulations for Medical Device Registration Medical Devices In Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Medical devices include a wide range of products. The chart illustrates the tga approval process per device classification in australia and is available for download. Provides a national system to control the quality, safety, efficacy and timely availability of therapeutic goods (including pharmaceuticals,. The argmd provides information. Medical Devices In Australia.
From www.australianmanufacturing.com.au
Australia’s first medical device development and manufacturing facility Medical Devices In Australia The argmd provides information on the import, export and supply of medical devices within australia. They include things like surgical. The medical devices market market in australia is forecasted to achieve a revenue of aud us$6.91bn in 2024. The australian tga medical device approval process explained. Medical devices include a wide range of products. Medical devices can be used to. Medical Devices In Australia.